当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polydopamine-Based Multifunctional Antitumor Nanoagent for Phototherapy and Photodiagnosis by Regulating Redox Balance
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-12-06 , DOI: 10.1021/acsabm.0c01057
Jie Feng 1 , Jia-Lin Gao 1 , Ruo-Yu Zhang 1 , Wen-Xiu Ren 1 , Yu-Bin Dong 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-12-06 , DOI: 10.1021/acsabm.0c01057
Jie Feng 1 , Jia-Lin Gao 1 , Ruo-Yu Zhang 1 , Wen-Xiu Ren 1 , Yu-Bin Dong 1
Affiliation
|
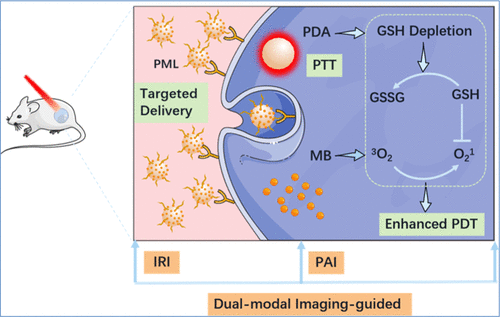
The development of multifunctional nanoagents for the simultaneous achievement of high diagnostic and therapeutic performances is significant for precise cancer treatment. Herein, we report on a polydopamine (PDA)-based multifunctional nanoagent, PML, in which the methylene blue (MB) photosensitizer (PS) and l-arginine (l-Arg) tumor-targeting species are equipped. After selectively accumulating in tumor sites, glutathione (GSH)-responsive PML degradation can controllably release loaded MB to produce singlet oxygen (1O2) under near-infrared (NIR) photoirradiation. This GSH-depleted PS release process can not only weaken the body’s antioxidant defence ability but also synergistically increase the 1O2 concentration. Therefore, GSH depletion-enhanced photodynamic therapy (PDT) efficiency is logically achieved by regulating the intracellular redox balance. In addition, our nanoagent can guide photoacoustic/NIR thermal dual-modal imaging and convert light into heat for cooperative cancer phototherapy because of the inherent photothermal conversion nature of PDA. As a result, excellent in vivo antitumor phototherapy (PDT + PTT) is achieved under the precise guidance of dual-modal imaging. This work not only realizes the integration of cancer diagnosis and treatment through PDA-based nanocarriers but also delivers dimensions in designing the next generation of multifunctional antitumor nanoagents for enhanced phototherapy and photodiagnosis by regulating the redox balance.
中文翻译:

多巴胺基多功能抗肿瘤纳米剂通过调节氧化还原平衡进行光疗和光诊断
开发用于同时实现高诊断和治疗性能的多功能纳米药物对于精确的癌症治疗具有重要意义。在此,我们报道了一种基于聚多巴胺 (PDA) 的多功能纳米制剂PML,其中配备了亚甲蓝 (MB) 光敏剂 (PS) 和l-精氨酸 ( l -Arg) 肿瘤靶向物质。在肿瘤部位选择性积累后,谷胱甘肽(GSH)响应的PML降解可以可控释放负载的MB产生单线态氧(1 O 2) 在近红外 (NIR) 光辐照下。这种GSH耗尽的PS释放过程不仅可以削弱人体的抗氧化防御能力,还可以协同增加1 O 2浓度。因此,GSH耗竭增强光动力疗法(PDT)效率是通过调节细胞内氧化还原平衡来实现的。此外,由于 PDA 固有的光热转换特性,我们的纳米剂可以引导光声/NIR 热双模成像并将光转化为热用于协同癌症光疗。结果,在体内表现出色抗肿瘤光疗(PDT+PTT)是在双模影像的精准引导下实现的。这项工作不仅通过基于PDA的纳米载体实现了癌症诊断和治疗的整合,而且为设计下一代多功能抗肿瘤纳米剂提供了维度,通过调节氧化还原平衡来增强光疗和光诊断。
更新日期:2020-12-21
中文翻译:

多巴胺基多功能抗肿瘤纳米剂通过调节氧化还原平衡进行光疗和光诊断
开发用于同时实现高诊断和治疗性能的多功能纳米药物对于精确的癌症治疗具有重要意义。在此,我们报道了一种基于聚多巴胺 (PDA) 的多功能纳米制剂PML,其中配备了亚甲蓝 (MB) 光敏剂 (PS) 和l-精氨酸 ( l -Arg) 肿瘤靶向物质。在肿瘤部位选择性积累后,谷胱甘肽(GSH)响应的PML降解可以可控释放负载的MB产生单线态氧(1 O 2) 在近红外 (NIR) 光辐照下。这种GSH耗尽的PS释放过程不仅可以削弱人体的抗氧化防御能力,还可以协同增加1 O 2浓度。因此,GSH耗竭增强光动力疗法(PDT)效率是通过调节细胞内氧化还原平衡来实现的。此外,由于 PDA 固有的光热转换特性,我们的纳米剂可以引导光声/NIR 热双模成像并将光转化为热用于协同癌症光疗。结果,在体内表现出色抗肿瘤光疗(PDT+PTT)是在双模影像的精准引导下实现的。这项工作不仅通过基于PDA的纳米载体实现了癌症诊断和治疗的整合,而且为设计下一代多功能抗肿瘤纳米剂提供了维度,通过调节氧化还原平衡来增强光疗和光诊断。




































 京公网安备 11010802027423号
京公网安备 11010802027423号