Tetrahedron ( IF 2.1 ) Pub Date : 2020-12-06 , DOI: 10.1016/j.tet.2020.131827 Sándor Nagy , Áron Szigetvári , Viktor Ilkei , Balázs Krámos , Zoltán Béni , Csaba Szántay , László Hazai
|
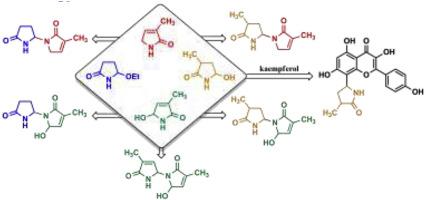
We hereby report the synthesis of six racemic alkaloids isolated from Lilium candidum L. Their common structural feature is a five-membered lactam ring which is, in the case of the flavonoid alkaloid lilaline, attached to the molecule’s aromatic core, while in the case of the other five compounds, it is connected to the nitrogen atom of a pyrrolinone ring by an aminal function. The syntheses of these natural products were achieved via Mannich-type alkylations through cyclic N-acyliminium ions as intermediates. Besides the synthesis, the so far unexplored stereochemistry of these natural products was determined by a combination of NMR-based proton–proton distance measurements and theoretical conformational analyses carried out at the DFT level.
中文翻译:

氨基酸型念珠菌百合生物碱和百合碱的合成;NMR光谱和DFT构象分析的协同使用确定它们的相对构型
我们据此报道了从假丝百合中分离出的六个外消旋生物碱的合成。它们的共同结构特征是五元内酰胺环,在黄酮类生物碱lalaline的情况下,其附着在分子的芳香核上,而在其他五种化合物则通过氨基功能连接到吡咯烷酮环的氮原子上。这些天然产物的合成是通过曼尼希型烷基化反应,以环状N-酰基亚胺离子为中间体。除了合成以外,这些天然产物迄今尚未探索的立体化学是通过基于NMR的质子-质子距离测量和在DFT级进行的理论构象分析相结合来确定的。













































 京公网安备 11010802027423号
京公网安备 11010802027423号