当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen abstraction of alkyl radicals from polycyclic aromatic hydrocarbons and heterocyclic aromatic hydrocarbons
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.ces.2020.116342 Yu-Hui Wang , Li-Tao Wang , Zhi-Zhen Yao , Jun-Jian Yin , Zi-Bin Huang , Pei-Qing Yuan , Wei-Kang Yuan
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.ces.2020.116342 Yu-Hui Wang , Li-Tao Wang , Zhi-Zhen Yao , Jun-Jian Yin , Zi-Bin Huang , Pei-Qing Yuan , Wei-Kang Yuan
|
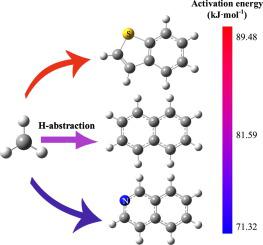
Abstract To understand the condensation during heavy oil thermal processing, the formation of aromatic carbon radicals by H-abstraction of methyl radicals (∙CH3) or ethyl radicals (∙C2H5) from polycyclic aromatic hydrocarbons (PAHs) or heterocyclic aromatic hydrocarbons (hetero-PAHs) was studied by density functional calculation. The H-abstraction from different PAHs all has a positive standard state Gibbs free energy change, while increasing reaction temperature or radical concentration promotes the reaction to proceed spontaneously. ∙CH3 mainly contributes to the formation of aromatic carbon radicals on PAHs, and the activation entropy of H-abstraction determines the difference in reaction rate constants. The particularity of H-abstraction from hetero-PAHs is reflected at the α-site of heterocycles. The H-abstraction from N-containing hetero-PAHs occurs preferentially at the α-site of heterocycles, and both ∙CH3 and ∙C2H5 could participate in abstracting the α-H. The H-abstraction from S-containing hetero-PAHs occurs preferentially on coupled aromatic rings, showing reaction behavior similar to the H-abstraction on PAHs.
中文翻译:

从多环芳烃和杂环芳烃中夺取烷基的氢
摘要 为了解重油热加工过程中的缩合,多环芳烃 (PAHs) 或杂环芳烃 (hetero-PAHs) 中甲基 (∙CH3) 或乙基 (∙C2H5) 的 H-抽提形成芳香碳自由基。 ) 通过密度泛函计算进行研究。来自不同PAHs的H-抽提均具有正的标准态吉布斯自由能变化,而提高反应温度或自由基浓度促进反应自发进行。∙CH3 主要有助于 PAHs 上芳香碳自由基的形成,H-抽象的活化熵决定了反应速率常数的差异。从杂多环芳烃中提取 H 的特殊性反映在杂环的 α 位点。含氮杂多环芳烃的 H 提取优先发生在杂环的 α 位点,∙CH3 和 ∙C2H5 都可以参与提取 α-H。含硫杂多环芳烃的 H-抽提优先发生在偶联的芳环上,反应行为类似于 PAH 上的 H-抽提。
更新日期:2021-03-01
中文翻译:

从多环芳烃和杂环芳烃中夺取烷基的氢
摘要 为了解重油热加工过程中的缩合,多环芳烃 (PAHs) 或杂环芳烃 (hetero-PAHs) 中甲基 (∙CH3) 或乙基 (∙C2H5) 的 H-抽提形成芳香碳自由基。 ) 通过密度泛函计算进行研究。来自不同PAHs的H-抽提均具有正的标准态吉布斯自由能变化,而提高反应温度或自由基浓度促进反应自发进行。∙CH3 主要有助于 PAHs 上芳香碳自由基的形成,H-抽象的活化熵决定了反应速率常数的差异。从杂多环芳烃中提取 H 的特殊性反映在杂环的 α 位点。含氮杂多环芳烃的 H 提取优先发生在杂环的 α 位点,∙CH3 和 ∙C2H5 都可以参与提取 α-H。含硫杂多环芳烃的 H-抽提优先发生在偶联的芳环上,反应行为类似于 PAH 上的 H-抽提。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号