当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of the Enzyme–Carrier Interface to Overcome the O2 and NADH Mass Transfer Limitations of an Immobilized Flavin Oxidase
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-12-04 , DOI: 10.1021/acsami.0c17568 Ana I. Benítez-Mateos 1, 2, 3 , Christina Huber 4 , Bernd Nidetzky 4, 5 , Juan M. Bolivar 4, 6 , Fernando López-Gallego 1, 2, 7
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-12-04 , DOI: 10.1021/acsami.0c17568 Ana I. Benítez-Mateos 1, 2, 3 , Christina Huber 4 , Bernd Nidetzky 4, 5 , Juan M. Bolivar 4, 6 , Fernando López-Gallego 1, 2, 7
Affiliation
|
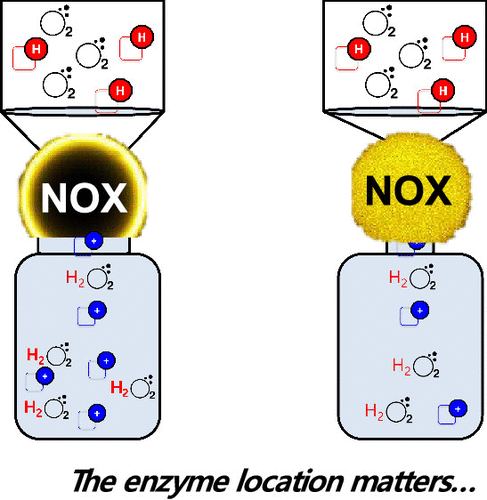
Understanding how the immobilization of enzymes on solid carriers affects their performance is paramount for the design of highly efficient heterogeneous biocatalysts. An efficient supply of substrates onto the solid phase is one of the main challenges to maximize the activity of the immobilized enzymes. Herein, we apply advanced single-particle analysis to decipher the optimal design of an immobilized NADH oxidase (NOX) whose activity depends both on O2 and NADH concentrations. Carrier physicochemical properties and its functionality along with the enzyme distribution across the carrier were implemented as design variables to study the effects of the intraparticle concentration of substrates (O2 and NADH) on the activity. Intraparticle O2-sensing analysis revealed the superior performance of the enzyme immobilized at the outer surface in terms of effective supply of O2. Furthermore, the co-immobilization of NADH and NOX within the tuned surface of porous microbeads increases the effective concentration of NADH in the surroundings of the enzyme. As a result, the optimal spatial organization of NOX and its confinement with NADH allow a 100% recovery of the activity of the soluble enzyme upon the immobilization process. By engineering these variables, we increase the NADH oxidation activity of the heterogeneous biocatalyst by up to 650% compared to NOX immobilized under suboptimal conditions. In conclusion, this work highlights the rational design and engineering of the enzyme–carrier interface to maximize the efficiency of heterogeneous biocatalysts.
中文翻译:

克服固定化黄素氧化酶的O 2和NADH传质限制的酶-载体界面设计
对于高效异质生物催化剂的设计,了解酶在固体载体上的固定化如何影响其性能至关重要。有效地将底物供应到固相上是使固定化酶的活性最大化的主要挑战之一。在这里,我们应用先进的单颗粒分析来破译固定化NADH氧化酶(NOX)的最佳设计,其活性取决于O 2和NADH浓度。载体的理化性质及其功能以及酶在整个载体中的分布均作为设计变量来研究底物(O 2和NADH)的颗粒内浓度对活性的影响。颗粒内O 2传感分析表明,固定在外表面的酶在有效供应O 2方面具有优越的性能。此外,将NADH和NOX共同固定在多孔微珠的调节表面内,可提高酶周围环境中NADH的有效浓度。结果,NOX的最佳空间组织及其在NADH中的封闭使得固定过程中可溶性酶的活性得以100%恢复。通过设计这些变量,与在次优条件下固定的NOX相比,我们将多相生物催化剂的NADH氧化活性提高了650%。总之,这项工作突出了酶-载体界面的合理设计和工程设计,以最大限度地提高非均相生物催化剂的效率。
更新日期:2020-12-16
中文翻译:

克服固定化黄素氧化酶的O 2和NADH传质限制的酶-载体界面设计
对于高效异质生物催化剂的设计,了解酶在固体载体上的固定化如何影响其性能至关重要。有效地将底物供应到固相上是使固定化酶的活性最大化的主要挑战之一。在这里,我们应用先进的单颗粒分析来破译固定化NADH氧化酶(NOX)的最佳设计,其活性取决于O 2和NADH浓度。载体的理化性质及其功能以及酶在整个载体中的分布均作为设计变量来研究底物(O 2和NADH)的颗粒内浓度对活性的影响。颗粒内O 2传感分析表明,固定在外表面的酶在有效供应O 2方面具有优越的性能。此外,将NADH和NOX共同固定在多孔微珠的调节表面内,可提高酶周围环境中NADH的有效浓度。结果,NOX的最佳空间组织及其在NADH中的封闭使得固定过程中可溶性酶的活性得以100%恢复。通过设计这些变量,与在次优条件下固定的NOX相比,我们将多相生物催化剂的NADH氧化活性提高了650%。总之,这项工作突出了酶-载体界面的合理设计和工程设计,以最大限度地提高非均相生物催化剂的效率。































 京公网安备 11010802027423号
京公网安备 11010802027423号