Chemosphere ( IF 8.1 ) Pub Date : 2020-12-05 , DOI: 10.1016/j.chemosphere.2020.129207
Fangfang Ma , Hong-Bin Xie , Mingxue Li , Sainan Wang , Renyi Zhang , Jingwen Chen
|
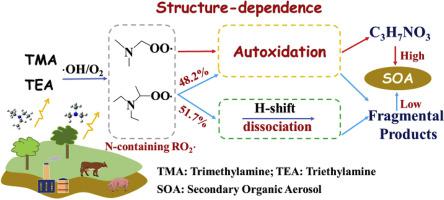
Tertiary amines are one kind of identified amines in the atmosphere. Here, the atmospheric oxidation mechanism and kinetics of tertiary amines were investigated by using computational methods. As proxies of these amines, trimethylamine (TMA) and triethylamine (TEA) have been selected. Results indicate that N-containing peroxy radicals (NRO2⋅), which are key intermediates in ⋅OH initiated oxidation of TMA and TEA, can follow a so-called autoxidation mechanism (a chain reaction of H-shift followed by O2 addition) even on the condition of high NO/HO2⋅ concentration. Such unique mechanism can be ascribed to the ability of N-atom in facilitating the unimolecular H-shift of NRO2⋅ and the absence of H-atoms on N-atom. However, different from TMA reaction system, the pathway dissociating into fragmental products can compete with the autoxidation pathway for TEA system. More importantly, TEA reaction system cannot lead to the formation of products with high O/C ratio due to the autoxidation pathway terminated by the release offragmental molecules. Such difference can be corroborated by previously observing lower secondary organic aerosol yield of TEA oxidation than that of TMA oxidation. The unveiled mechanism enhances current understanding on atmospheric fate of amines and autoxidation mechanism.
中文翻译:

叔胺大气氧化的自氧化机理:对次级有机气溶胶形成的影响。
叔胺是大气中已识别的一种胺。在此,通过计算方法研究了叔胺的大气氧化机理和动力学。作为这些胺的代理,已经选择了三甲胺(TMA)和三乙胺(TEA)。结果表明含N的过氧自由基(NRO即2 ⋅),这是在⋅OH发起TMA和TEA氧化的关键中间体,可以按照一个所谓的自氧化机制(接着被O H-移的链反应2加法)即使在高NO / HO的条件2 ⋅浓度。这种独特的机制可以归因于N原子促进NRO 2的单分子H位移的能力。⋅和N原子上不存在H原子。然而,不同于TMA反应系统,分解成碎片产物的途径可以与TEA系统的自氧化途径竞争。更重要的是,由于自氧化途径被释放的杂乱分子所终止,TEA反应系统不能导致形成高O / C比的产物。通过事先观察到TEA氧化的二次有机气溶胶产量低于TMA氧化的二次有机气溶胶产量,可以证实这种差异。揭示的机理增强了当前对胺的大气命运和自氧化机理的理解。







































 京公网安备 11010802027423号
京公网安备 11010802027423号