当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Insecticidal Activity of 5,5-Disubstituted 4,5-Dihydropyrazolo[1,5-a]quinazolines as Novel Antagonists of GABA Receptors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-12-03 , DOI: 10.1021/acs.jafc.0c02462 Xunyuan Jiang 1 , Shuai Yang 1 , Ying Yan 1 , Fei Lin 1 , Ling Zhang 2 , Weijing Zhao 1 , Chen Zhao 1 , Hanhong Xu 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-12-03 , DOI: 10.1021/acs.jafc.0c02462 Xunyuan Jiang 1 , Shuai Yang 1 , Ying Yan 1 , Fei Lin 1 , Ling Zhang 2 , Weijing Zhao 1 , Chen Zhao 1 , Hanhong Xu 1
Affiliation
|
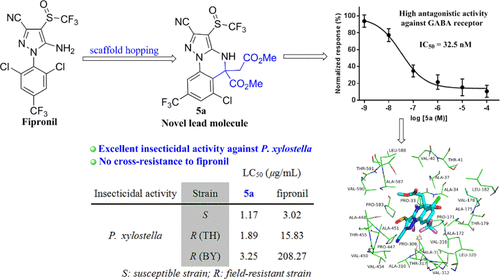
To control the development of resistance to conventional insecticides acting as γ-aminobutyric acid (GABA) receptor antagonists (e.g., fipronil), new GABAergic 5,5-disubstituted 4,5-dihydropyrazolo[1,5-a]quinazolines were designed via a scaffold-hopping strategy and synthesized with a facile method. Among the 50 target compounds obtained, compounds 5a, 5b, 7a, and 7g showed excellent insecticidal activities against a susceptible strain of Plutella xylostella (LC50 values ranging from 1.03 to 1.44 μg/mL), which were superior to that of fipronil (LC50 = 3.02 μg/mL). Remarkably, the insecticidal activity of compound 5a was 64-fold better than that of fipronil against the field population of fipronil-resistant P. xylostella. Electrophysiological studies against the housefly GABA receptor heterologously expressed in Xenopus oocytes indicated that compound 5a could act as a potent GABA receptor antagonist, and IC50 was calculated to be 32.5 nM. Molecular docking showed that the binding poses of compound 5a with the housefly GABA receptor can be different compared to fipronil, which explains the effectiveness of compound 5a against fipronil-resistant insects. These findings have suggested compound 5a as a lead compound for a novel GABA receptor antagonist controlling field-resistant insects and provided a basis for further design, structural modification, and development of 4,5-dihydropyrazolo[1,5-a]quinazoline motifs as new insecticidal GABA receptor antagonists.
中文翻译:

5,5-二取代的4,5-二氢吡唑并[1,5- a ]喹唑啉类化合物作为GABA受体新型拮抗剂的设计,合成及杀虫活性
为了控制对用作γ-氨基丁酸(GABA)受体拮抗剂(例如,氟虫腈)的常规杀虫剂的抗性发展,设计了一种新的GABA能5,5-二取代4,5-二氢吡唑并[1,5- a ]喹唑啉。脚手架跳跃策略并采用简便的方法进行合成。在获得的50种目标化合物中,化合物5a,5b,7a和7g对小菜蛾敏感菌株表现出优异的杀虫活性(LC 50值为1.03至1.44μg/ mL),优于氟虫腈(LC 50 = 3.02μg/ mL)。显着地,化合物5a的杀虫活性对于耐氟虫腈的小菜蛾田间种群,其抗性比氟虫腈高64倍。针对非洲爪蟾卵母细胞中异源表达的家蝇GABA受体的电生理研究表明,化合物5a可以作为有效的GABA受体拮抗剂,IC 50计算为32.5 nM。分子对接表明,化合物5a与家蝇GABA受体的结合姿势与氟虫腈相比可能有所不同,这说明了化合物5a对耐氟虫腈的昆虫有效。这些发现表明化合物5a作为控制田间昆虫的新型GABA受体拮抗剂的先导化合物,并为进一步设计,结构修饰和开发作为新型杀虫GABA受体拮抗剂的4,5-二氢吡唑并[1,5- a ]喹唑啉基序提供了基础。
更新日期:2020-12-16
中文翻译:

5,5-二取代的4,5-二氢吡唑并[1,5- a ]喹唑啉类化合物作为GABA受体新型拮抗剂的设计,合成及杀虫活性
为了控制对用作γ-氨基丁酸(GABA)受体拮抗剂(例如,氟虫腈)的常规杀虫剂的抗性发展,设计了一种新的GABA能5,5-二取代4,5-二氢吡唑并[1,5- a ]喹唑啉。脚手架跳跃策略并采用简便的方法进行合成。在获得的50种目标化合物中,化合物5a,5b,7a和7g对小菜蛾敏感菌株表现出优异的杀虫活性(LC 50值为1.03至1.44μg/ mL),优于氟虫腈(LC 50 = 3.02μg/ mL)。显着地,化合物5a的杀虫活性对于耐氟虫腈的小菜蛾田间种群,其抗性比氟虫腈高64倍。针对非洲爪蟾卵母细胞中异源表达的家蝇GABA受体的电生理研究表明,化合物5a可以作为有效的GABA受体拮抗剂,IC 50计算为32.5 nM。分子对接表明,化合物5a与家蝇GABA受体的结合姿势与氟虫腈相比可能有所不同,这说明了化合物5a对耐氟虫腈的昆虫有效。这些发现表明化合物5a作为控制田间昆虫的新型GABA受体拮抗剂的先导化合物,并为进一步设计,结构修饰和开发作为新型杀虫GABA受体拮抗剂的4,5-二氢吡唑并[1,5- a ]喹唑啉基序提供了基础。









































 京公网安备 11010802027423号
京公网安备 11010802027423号