Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-12-03 , DOI: 10.1016/j.jhazmat.2020.124748
Zhao Wang , Junfeng Su , Xiaofen Hu , Amjad Ali , Zizhen Wu
|
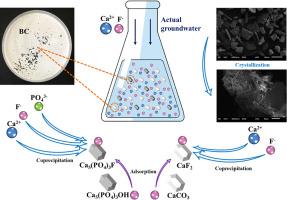
Biosynthetic crystals (BC) were prepared through microbially induced calcium carbonate precipitation (MICP) for fluoride (F-) removal from the groundwater. Batch experiments were conducted to evaluate the fluoride adsorption capacity and the impacts of critical factors (organic matter, pH, initial fluoride concentration and BC dosage) on defluorination efficiency of BC. The maximum adsorption amount and defluorination efficiency were recorded as 5.10 mg g-1 and 98.24%, respectively. The adsorption kinetics and isotherms studies showed that pseudo-second-order kinetic model and Freundlich isotherm model were best fitting to the reaction. Adsorption thermodynamic parameters indicated a spontaneous, endothermic and thermodynamically favorable adsorption process. Moreover, the mechanism of F- removal by BC was further analyzed by SEM, XPS, XRD and FTIR. The method can cope with the problem of applying the external organic substances in MICP, and avoid the microbial safety risk in the effluent. As an economically and environmentally friendly adsorbent, BC can be used for F- removal from groundwater.
中文翻译:

微生物诱导的碳酸钙沉淀法分离生物合成晶体及其在地下水中除氟的应用
生物合成晶体(BC)通过微生物引起的碳酸钙沉淀(MICP)氟化物(F制备-从地下水去除)。进行批处理实验以评估氟化物的吸附能力以及关键因素(有机物,pH,初始氟化物浓度和BC剂量)对BC脱氟效率的影响。记录最大吸附量和脱氟效率为5.10 mg g -1和98.24%。吸附动力学和等温线研究表明,拟二级动力学模型和Freundlich等温线模型最适合该反应。吸附热力学参数表明自发的,吸热的和热力学上有利的吸附过程。此外,F的机构-通过去除BC通过SEM,XPS,X射线衍射和FTIR进一步分析。该方法可以解决在MICP中使用外部有机物的问题,并避免了污水中微生物的安全隐患。作为一种经济和环境友好的吸附剂,BC,可以使用适用于F -地下水去除。

































 京公网安备 11010802027423号
京公网安备 11010802027423号