European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-12-03 , DOI: 10.1016/j.ejmech.2020.113045 Katja Silbermann , Jiyang Li , Vigneshwaran Namasivayam , Sven Marcel Stefan , Michael Wiese
|
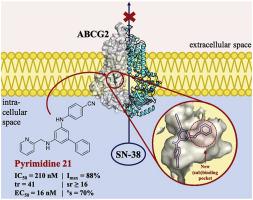
In the search for novel, highly potent, and nontoxic adjuvant chemotherapeutics to resolve the major issue of ABC transporter-mediated multidrug resistance (MDR), pyrimidines were discovered as a promising compound class of modern ABCG2 inhibitors. As ABCG2-mediated MDR is a major obstacle in leukemia, pancreatic carcinoma, and breast cancer chemotherapy, adjuvant chemotherapeutics are highly desired for future clinical oncology. Very recently, docking studies of one of the most potent reversers of ABCG2-mediated MDR were reported and revealed a putative second binding pocket of ABCG2. Based on this (sub)pocket, a series of 16 differently 6-substituted 4-anilino-2-phenylpyrimidines were synthesized to explore the potential increase in inhibitory activity of these ABCG2 inhibitors. The compounds were assessed for their influence on the ABCG2-mediated pheophorbide A transport, as well as the ABCB1- and ABCC1-mediated transport of calcein AM. They were additionally evaluated in MDR reversal assays to determine their half-maximal reversal concentration (EC50). The 6-substitution did not only show increased toxicity against ABCG2-overexpressing cells in combination with SN-38 but also a negative influence on cell viability in general. Nevertheless, several candidates have EC50 values in the low double-digit nanomolar concentration range, qualifying them as some of the most potent reversers of ABCG2-mediated MDR. In addition, five novel multitarget ABCB1/ABCC1/ABCG2 inhibitors were discovered, four of them exerting their inhibitory power against the three stated transporters at least in the single-digit micromolar concentration range.
中文翻译:

探索新的ABCG2结合位点的6取代的4-苯胺基-2-苯基嘧啶的合理药物设计
在寻找新颖,高效且无毒的辅助化学疗法以解决ABC转运蛋白介导的多药耐药性(MDR)的主要问题时,发现嘧啶是现代ABCG2抑制剂的有前途的化合物类别。由于ABCG2介导的MDR是白血病,胰腺癌和乳腺癌化学疗法的主要障碍,因此,对于未来的临床肿瘤学,非常需要辅助化学疗法。最近,报道了对最有效的ABCG2介导的MDR逆转剂之一的对接研究,揭示了ABCG2的第二个潜在结合口袋。基于这个(子)口袋,合成了16种不同的6个取代的4-苯胺基-2-苯基嘧啶系列,以探索这些ABCG2抑制剂抑制活性的潜在增加。评估了这些化合物对ABCG2介导的脱镁叶绿酸A转运以及ABCB1和ABCC1介导的钙黄绿素AM的影响。另外在MDR逆转分析中对它们进行了评估,以确定其半最大逆转浓度(EC50)。6取代不仅与SN-38结合使用,对ABCG2过表达细胞的毒性增加,而且总体上对细胞活力也有负面影响。尽管如此,仍有几位候选者的EC 50值处于较低的两位数纳摩尔浓度范围内,从而使它们成为ABCG2介导的MDR的最有效反向剂。此外,发现了五种新颖的多靶点ABCB1 / ABCC1 / ABCG2抑制剂,其中四种抑制剂至少在单位微摩尔浓度范围内对三种所述转运蛋白发挥了抑制作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号