当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Aerobic Decomposition of 1,3-Diaryl-2-diazo-1,3-diketones: Mechanistic Investigation and Application to the Synthesis of Benzils
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-02 , DOI: 10.1021/acs.joc.0c02366 Jia-Liang Zhu, Yi-Ting Tsai
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-02 , DOI: 10.1021/acs.joc.0c02366 Jia-Liang Zhu, Yi-Ting Tsai
|
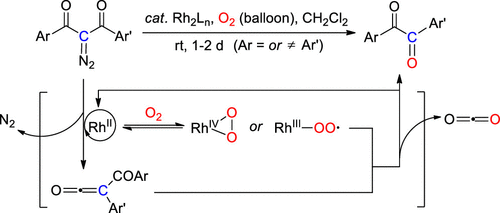
The conversion of 1,3-diaryl-2-diazo-1,3-diketones to 1,2-daryl-1,2-diketones (benzils) is reported based on a rhodium(II)-catalyzed aerobic decomposition process. The reaction occurs at ambient temperatures and can be catalyzed by a few dirhodium carboxylates (5 mol %) under a balloon pressure of oxygen. Moreover, an oxygen atom from the O2 reagent is shown to be incorporated into the product, and this is accompanied by the extrusion of a carbonyl unit from the starting materials. Mechanistically, it is proposed that the decomposition may proceed via the interaction of a ketene intermediate resulting from a Wolff rearrangement of the carbenoid, with a rhodium peroxide or peroxy radical species generated upon the activation of molecular oxygen. The proposed mechanism has been supported by the results from a set of controlled experiments. By using this newly developed strategy, a large array of benzil derivatives as well as 9,10-phenanthrenequinone were synthesized from the corresponding diazo substrates in varying yields. On the other hand, the method did not allow the generation of benzocyclobutene-1,2-dione from 2-diazo-1,3-indandione because of the difficulty of inducing the initial rearrangement.
中文翻译:

1,3-二芳基-2-重氮-1,3-二酮的铑催化好氧分解:机理研究及其在苯合成中的应用
基于铑(II)催化的需氧分解过程,报道了1,3-二芳基-2-重氮-1,3-二酮向1,2-二芳基-1,2-二酮(苯)的转化。该反应在环境温度下发生,并且可以在氧气的气球压力下由少量羧酸的铑(5mol%)催化。此外,显示出来自O 2试剂的氧原子被掺入产物中,并且这伴随着羰基单元从起始原料的挤出。从机理上讲,建议分解可以通过类胡萝卜素的沃尔夫重排产生的乙烯酮中间体与分子氧活化后产生的过氧化铑或过氧自由基的相互作用。一组受控实验的结果支持了所提出的机制。通过使用这种新开发的策略,从相应的重氮底物以不同的收率合成了大量苯并衍生物和9,10-菲醌。另一方面,由于难以诱导初始重排,该方法不能从2-重氮-1,3-茚满二酮生成苯并环丁烯-1,2-二酮。
更新日期:2021-01-01
中文翻译:

1,3-二芳基-2-重氮-1,3-二酮的铑催化好氧分解:机理研究及其在苯合成中的应用
基于铑(II)催化的需氧分解过程,报道了1,3-二芳基-2-重氮-1,3-二酮向1,2-二芳基-1,2-二酮(苯)的转化。该反应在环境温度下发生,并且可以在氧气的气球压力下由少量羧酸的铑(5mol%)催化。此外,显示出来自O 2试剂的氧原子被掺入产物中,并且这伴随着羰基单元从起始原料的挤出。从机理上讲,建议分解可以通过类胡萝卜素的沃尔夫重排产生的乙烯酮中间体与分子氧活化后产生的过氧化铑或过氧自由基的相互作用。一组受控实验的结果支持了所提出的机制。通过使用这种新开发的策略,从相应的重氮底物以不同的收率合成了大量苯并衍生物和9,10-菲醌。另一方面,由于难以诱导初始重排,该方法不能从2-重氮-1,3-茚满二酮生成苯并环丁烯-1,2-二酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号