当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Efficient and Selective Hg(II) Removal from Water Using Multilayered Ti3C2Ox MXene via Adsorption Coupled with Catalytic Reduction Mechanism
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.est.0c05532 Kaixing Fu 1 , Xia Liu 1 , Deyou Yu 2 , Jinming Luo 2, 3 , Zhiwei Wang 4 , John C. Crittenden 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.est.0c05532 Kaixing Fu 1 , Xia Liu 1 , Deyou Yu 2 , Jinming Luo 2, 3 , Zhiwei Wang 4 , John C. Crittenden 2
Affiliation
|
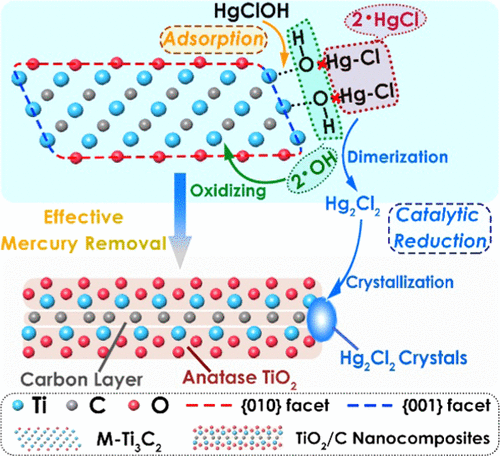
Mercury (Hg) removal is crucial to the safety of water resources, yet it lacks an effective removal technology, especially for emergency on-site remediation. Herein, multilayered oxygen-functionalized Ti3C2 (Ti3C2Ox) (abbreviated as M-Ti3C2) nanosheets were prepared to remove Hg(II) from water. The M-Ti3C2 has demonstrated ultrafast adsorption kinetics (the concentration decreased from 10 400 to 33 μg L–1 in 10 s), impressively high capacity (4806 mg g–1), high selectivity, and broad working pH range (3–12). The density functional theory (DFT) calculations and experimental characterizations unveil that this exceptional Hg(II) removal is owing to the distinct interaction (e.g., adsorption coupled with catalytic reduction). Specifically, Ti atoms on the {001} facets of M-Ti3C2 prefer to adsorb Hg(II) in the form of HgClOH, which subsequently undergoes homolytic cleavage to form radical species (e.g., •OH and •HgCl). Immediately, the •HgCl radicals dimerize and form crystalline Hg2Cl2 on the edges of M-Ti3C2. Up to ∼95% of dimeric Hg2Cl2 can be efficiently recovered via facile thermal treatment. Notably, owing to the adsorbed •OH and energy released during the distinct interaction, M-Ti3C2 has been oxidized to TiO2/C nanocomposites. And the TiO2/C nanocomposites have shown to have better performance on the photocatalytic degradation of organic pollutants than Degussa P25. These exceptional features coupled with mercuric recyclable nature make M-Ti3C2 an outstanding candidate for rapid/urgent Hg(II) removal and recovery.
中文翻译:

多层Ti 3 C 2 O x MXene通过吸附与催化还原机理高效高效地去除水中的Hg(II)
汞(Hg)的去除对于水资源安全至关重要,但是它缺乏有效的去除技术,尤其是对于紧急现场修复。在此,制备了多层的氧官能化的Ti 3 C 2(Ti 3 C 2 O x)(缩写为M-Ti 3 C 2)纳米片,以从水中去除Hg(II)。M-Ti 3 C 2具有超快的吸附动力学(浓度在10 s内从10 400降至33μgL –1),超高容量(4806 mg g –1)),高选择性和宽的工作pH范围(3-12)。密度泛函理论(DFT)的计算和实验表征表明,这种特殊的Hg(II)去除是由于独特的相互作用(例如,吸附与催化还原相结合)。具体地,在M-Ti中的{001}面Ti原子3 Ç 2在HgClOH的形式,其随后经历均裂形成自由基物种偏爱以吸附汞(II)(例如,• OH和•升汞)。随即,•的HgCl基团二聚化和形式结晶汞柱2氯2上M-Ti中的边缘3 Ç 2。通过简便的热处理可以有效回收高达约95%的Hg 2 Cl 2二聚体。值得注意的是,由于在不同相互作用期间吸附的• OH和释放的能量,M-Ti 3 C 2已被氧化为TiO 2 / C纳米复合材料。TiO 2 / C纳米复合材料在光催化降解有机污染物方面具有比Degussa P25更好的性能。这些优异的特性加上汞可循环利用的性质,使得M-Ti 3 C 2成为快速/紧急去除和回收Hg(II)的杰出候选者。
更新日期:2020-12-15
中文翻译:

多层Ti 3 C 2 O x MXene通过吸附与催化还原机理高效高效地去除水中的Hg(II)
汞(Hg)的去除对于水资源安全至关重要,但是它缺乏有效的去除技术,尤其是对于紧急现场修复。在此,制备了多层的氧官能化的Ti 3 C 2(Ti 3 C 2 O x)(缩写为M-Ti 3 C 2)纳米片,以从水中去除Hg(II)。M-Ti 3 C 2具有超快的吸附动力学(浓度在10 s内从10 400降至33μgL –1),超高容量(4806 mg g –1)),高选择性和宽的工作pH范围(3-12)。密度泛函理论(DFT)的计算和实验表征表明,这种特殊的Hg(II)去除是由于独特的相互作用(例如,吸附与催化还原相结合)。具体地,在M-Ti中的{001}面Ti原子3 Ç 2在HgClOH的形式,其随后经历均裂形成自由基物种偏爱以吸附汞(II)(例如,• OH和•升汞)。随即,•的HgCl基团二聚化和形式结晶汞柱2氯2上M-Ti中的边缘3 Ç 2。通过简便的热处理可以有效回收高达约95%的Hg 2 Cl 2二聚体。值得注意的是,由于在不同相互作用期间吸附的• OH和释放的能量,M-Ti 3 C 2已被氧化为TiO 2 / C纳米复合材料。TiO 2 / C纳米复合材料在光催化降解有机污染物方面具有比Degussa P25更好的性能。这些优异的特性加上汞可循环利用的性质,使得M-Ti 3 C 2成为快速/紧急去除和回收Hg(II)的杰出候选者。































 京公网安备 11010802027423号
京公网安备 11010802027423号