当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Techno-Economic Analysis of Integrating a CO2 Hydrogenation-to-Methanol Unit with a Coal-to-Methanol Process for CO2 Reduction
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-12-02 , DOI: 10.1021/acssuschemeng.0c06336
Jingpeng Zhang 1 , Zhengwen Li 1 , Zhihe Zhang 1 , Rong Liu 2 , Bozhao Chu 3 , Binhang Yan 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-12-02 , DOI: 10.1021/acssuschemeng.0c06336
Jingpeng Zhang 1 , Zhengwen Li 1 , Zhihe Zhang 1 , Rong Liu 2 , Bozhao Chu 3 , Binhang Yan 1
Affiliation
|
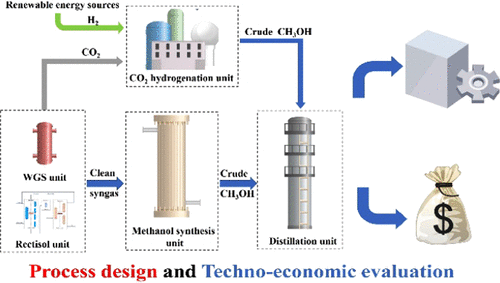
A coal-to-methanol (CTM) process is one of the great important industrial methanol production processes, which has attracted a lot of attention in light of dwindling petroleum sources and rising prices of natural gas and oil. However, the CTM process will typically result in a certain amount of CO2 emission and thereafter lead to severe environmental problems. Facing historic global warming, a carbon emissions trading system drives the CTM process to adopt CO2 reduction technologies. In this paper, a CO2 hydrogenation-to-methanol unit, regarded as one of the most promising technology for CO2 reduction, is integrated with the CTM process to achieve CO2 reduction under the carbon emissions trading system. A detailed simulation of the CTM process integrated with the CO2 hydrogenation-to-methanol unit is built based on a typical industrial process. Mass and energy balance results indicate that the CTM process without a CO2 reduction unit emits 3.1 kg of CO2·kg CH3OH–1. The CO2 hydrogenation-to-methanol unit, consuming 0.12 kg of H2 and 7.4 MJ of energy, could achieve a net CO2 reduction with H2-associated CO2 emission below 1.8 kg of CO2·kg H2–1. The energy efficiency of the CTM process only slightly decreases from 52.9 to 51.6% when the carbon cap drops from 3.1 to 2 kg of CO2·kg CH3OH–1. Furthermore, an overall profit could be obtained with the CO2 hydrogenation unit when the H2 price is under the critical point at 1.4 US$·kg H2–1.
中文翻译:

整合一个CO的技术经济分析2与煤制甲醇过程CO加氢至甲醇装置2还原
煤制甲醇(CTM)工艺是重要的工业甲醇生产工艺之一,鉴于石油资源的减少以及天然气和石油价格的上涨,吸引了很多关注。但是,CTM工艺通常会导致一定量的CO 2排放,此后会导致严重的环境问题。面对历史性的全球变暖,碳排放交易系统推动CTM工艺采用CO 2减排技术。在本文中,CO 2加氢制甲醇装置被认为是最有前途的CO 2还原技术之一,它与CTM工艺集成以实现CO 2在碳排放权交易制度下的减排。基于典型的工业过程,建立了与CO 2加氢制甲醇装置集成的CTM工艺的详细模拟。质量和能量平衡结果表明,不使用CO 2还原装置的CTM工艺排放3.1 kg CO 2 ·kg CH 3 OH –1。CO 2加氢制甲醇装置消耗0.12 kg的H 2和7.4 MJ的能量,通过与H 2相关的CO 2排放低于1.8 kg CO 2 ·kg H 2 –1,可以实现净CO 2减少。。当碳含量从3.1 kg的CO 2 ·kg CH 3 OH –1降至2 kg时,CTM工艺的能源效率仅从52.9略微降低到51.6%。此外,当H 2价格在1.4美元·kg H 2 –1的临界点以下时,使用CO 2加氢装置可以获得整体利润。
更新日期:2020-12-14
中文翻译:

整合一个CO的技术经济分析2与煤制甲醇过程CO加氢至甲醇装置2还原
煤制甲醇(CTM)工艺是重要的工业甲醇生产工艺之一,鉴于石油资源的减少以及天然气和石油价格的上涨,吸引了很多关注。但是,CTM工艺通常会导致一定量的CO 2排放,此后会导致严重的环境问题。面对历史性的全球变暖,碳排放交易系统推动CTM工艺采用CO 2减排技术。在本文中,CO 2加氢制甲醇装置被认为是最有前途的CO 2还原技术之一,它与CTM工艺集成以实现CO 2在碳排放权交易制度下的减排。基于典型的工业过程,建立了与CO 2加氢制甲醇装置集成的CTM工艺的详细模拟。质量和能量平衡结果表明,不使用CO 2还原装置的CTM工艺排放3.1 kg CO 2 ·kg CH 3 OH –1。CO 2加氢制甲醇装置消耗0.12 kg的H 2和7.4 MJ的能量,通过与H 2相关的CO 2排放低于1.8 kg CO 2 ·kg H 2 –1,可以实现净CO 2减少。。当碳含量从3.1 kg的CO 2 ·kg CH 3 OH –1降至2 kg时,CTM工艺的能源效率仅从52.9略微降低到51.6%。此外,当H 2价格在1.4美元·kg H 2 –1的临界点以下时,使用CO 2加氢装置可以获得整体利润。































 京公网安备 11010802027423号
京公网安备 11010802027423号