当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,5,7‐Triazabicyclo[4.4.0]dec‐5‐ene Enhances Activity of Peroxide Intermediates in Phosphine‐Free α‐Hydroxylation of Ketones
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-12-01 , DOI: 10.1002/anie.202014478 Yongtao Wang 1 , Rui Lu 1 , Jia Yao 2 , Haoran Li 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-12-01 , DOI: 10.1002/anie.202014478 Yongtao Wang 1 , Rui Lu 1 , Jia Yao 2 , Haoran Li 3
Affiliation
|
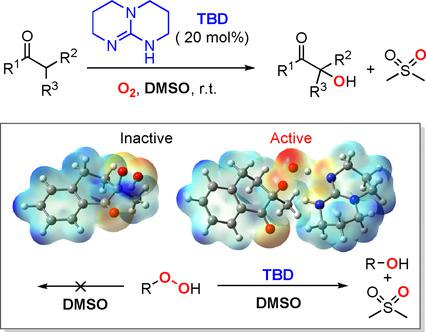
The critical role of double hydrogen bonds was addressed for the aerobic α‐hydroxylation of ketones catalyzed by 1,5,7‐triazabicyclo[4.4.0]dec‐5‐ene (TBD), in the absence of either a metal catalyst or phosphine reductant. Experimental and theoretical investigations were performed to study the mechanism. In addition to initiating the reaction by proton abstraction, a more important role of TBD was revealed, that is, to enhance the oxidizing ability of peroxide intermediates, allowing DMSO to be used rather than commonly used phosphine reductants. Further characterizations with nuclear Overhauser effect spectroscopy (NOESY) confirmed the presence of double hydrogen bonds between TBD and the ketone, and kinetic studies suggested the attack of dioxygen on the TBD‐enol adduct to be the rate‐determining step. This work should encourage the application of TBD as a catalyst for oxidations.
中文翻译:

1,5,7-三氮杂双环[4.4.0] dec-5-ene增强酮类无磷α-羟基氧化反应中的过氧化物中间体的活性
在没有金属催化剂或磷化氢的情况下,双氢键的关键作用已被1,5,7-三氮杂双环[4.4.0] dec-5-烯(TBD)催化的酮的好氧α-羟基化反应还原剂。进行了实验和理论研究以研究其机理。除了通过质子提取引发反应之外,还显示了TBD的更重要作用,即增强过氧化物中间体的氧化能力,从而允许使用DMSO而不是常用的膦还原剂。核Overhauser效应光谱(NOESY)的进一步表征证实了TBD和酮之间存在双氢键,动力学研究表明,TBD-烯醇加合物上的双氧攻击是决定速率的步骤。
更新日期:2020-12-01
中文翻译:

1,5,7-三氮杂双环[4.4.0] dec-5-ene增强酮类无磷α-羟基氧化反应中的过氧化物中间体的活性
在没有金属催化剂或磷化氢的情况下,双氢键的关键作用已被1,5,7-三氮杂双环[4.4.0] dec-5-烯(TBD)催化的酮的好氧α-羟基化反应还原剂。进行了实验和理论研究以研究其机理。除了通过质子提取引发反应之外,还显示了TBD的更重要作用,即增强过氧化物中间体的氧化能力,从而允许使用DMSO而不是常用的膦还原剂。核Overhauser效应光谱(NOESY)的进一步表征证实了TBD和酮之间存在双氢键,动力学研究表明,TBD-烯醇加合物上的双氧攻击是决定速率的步骤。































 京公网安备 11010802027423号
京公网安备 11010802027423号