当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hybrid Peptide–Thiourea Catalyst for Asymmetric Michael Additions of Aldehydes to Heterocyclic Nitroalkenes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.joc.0c02251 Patrícia Čmelová 1 , Denisa Vargová 1 , Radovan Šebesta 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.joc.0c02251 Patrícia Čmelová 1 , Denisa Vargová 1 , Radovan Šebesta 1
Affiliation
|
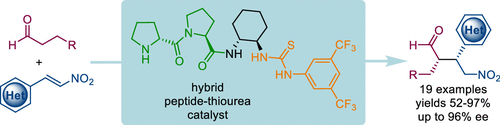
Bifunctional organocatalysis combining covalent and noncovalent activation is presented. The hybrid peptide–thiourea catalyst features a N-terminal proline moiety for aldehyde activation and a thiourea unit for electrophile activation. This catalyst effectively promotes asymmetric Michael additions of aldehydes to challenging but biologically relevant heterocycle-containing nitroalkenes. The catalyst can be used under solvent-free conditions. Spectroscopic and density functional theory studies elucidate the catalyst structure and mode of action.
中文翻译:

杂肽-硫脲催化醛与杂环硝基烯烃的不对称迈克尔加成反应
提出了结合共价和非共价活化的双功能有机催化。杂肽-硫脲催化剂具有用于醛活化的N末端脯氨酸部分和用于亲电试剂活化的硫脲单元。该催化剂有效地促进醛向具有挑战性但生物学上相关的含杂环硝基烯烃的醛的不对称迈克尔加成。该催化剂可以在无溶剂的条件下使用。光谱和密度泛函理论研究阐明了催化剂的结构和作用方式。
更新日期:2021-01-01
中文翻译:

杂肽-硫脲催化醛与杂环硝基烯烃的不对称迈克尔加成反应
提出了结合共价和非共价活化的双功能有机催化。杂肽-硫脲催化剂具有用于醛活化的N末端脯氨酸部分和用于亲电试剂活化的硫脲单元。该催化剂有效地促进醛向具有挑战性但生物学上相关的含杂环硝基烯烃的醛的不对称迈克尔加成。该催化剂可以在无溶剂的条件下使用。光谱和密度泛函理论研究阐明了催化剂的结构和作用方式。































 京公网安备 11010802027423号
京公网安备 11010802027423号