当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidation of Factors That Govern the 2e–/2H+ vs 4e–/4H+ Selectivity of Water Oxidation by a Cobalt Corrole
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-12-01 , DOI: 10.1021/jacs.0c08654 Biswajit Mondal 1 , Samir Chattopadhyay 1 , Subal Dey 1 , Atif Mahammed 2 , Kaustuv Mittra 1 , Atanu Rana 1 , Zeev Gross 2 , Abhishek Dey 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-12-01 , DOI: 10.1021/jacs.0c08654 Biswajit Mondal 1 , Samir Chattopadhyay 1 , Subal Dey 1 , Atif Mahammed 2 , Kaustuv Mittra 1 , Atanu Rana 1 , Zeev Gross 2 , Abhishek Dey 1
Affiliation
|
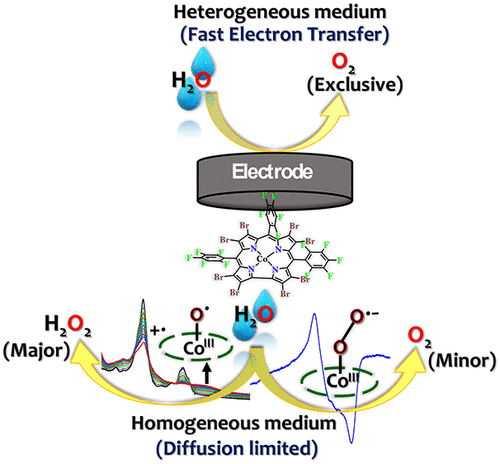
Considering the importance of water splitting as the best solution for clean and renewable energy, the worldwide efforts for development of increasingly active molecular water oxidation catalysts must be accompanied by studies that focus on elucidating the mode of actions and catalytic pathways. One crucial challenge remains the elucidation of the factors that determine the selectivity of water oxidation by the desired 4e-/4H+ pathway that leads to O2 rather than by 2e-/2H+ to H2O2. We now show that water oxidation with the cobalt-corrole CoBr8 as electrocatalyst affords H2O2 as the main product in homogeneous solutions, while heterogeneous water oxidation by the same catalyst leads exclusively to oxygen. Experimental and computation-based investigations of the species formed during the process uncover the formation of a Co(III)-superoxide intermediate and its preceding high-valent Co-oxyl complex. The competition between the base-catalyzed hydrolysis of Co(III)-hydroperoxide [Co(III)-OOH]- to release H2O2 and the electrochemical oxidation of the same to release O2 via [Co(III)-O2•]- is identified as the key step determining the selectivity of water oxidation.
中文翻译:

阐明控制钴腐蚀对水氧化的 2e–/2H+ 与 4e–/4H+ 选择性的因素
考虑到水分解作为清洁和可再生能源的最佳解决方案的重要性,世界范围内开发活性分子水氧化催化剂的努力必须伴随着重点阐明作用模式和催化途径的研究。一个关键的挑战仍然是阐明决定水氧化选择性的因素是通过所需的 4e-/4H+ 途径生成 O2 而不是通过 2e-/2H+ 生成 H2O2。我们现在表明,使用钴-钴 CoBr8 作为电催化剂进行水氧化,在均相溶液中提供 H2O2 作为主要产物,而由相同催化剂进行的非均相水氧化仅产生氧气。在该过程中形成的物种的实验和基于计算的研究揭示了 Co(III)-超氧化物中间体及其之前的高价 Co-oxyl 复合物的形成。确定了碱催化水解 Co(III)-氢过氧化物 [Co(III)-OOH]- 以释放 H2O2 与电化学氧化通过 [Co(III)-O2•]- 释放 O2 之间的竞争作为决定水氧化选择性的关键步骤。
更新日期:2020-12-01
中文翻译:

阐明控制钴腐蚀对水氧化的 2e–/2H+ 与 4e–/4H+ 选择性的因素
考虑到水分解作为清洁和可再生能源的最佳解决方案的重要性,世界范围内开发活性分子水氧化催化剂的努力必须伴随着重点阐明作用模式和催化途径的研究。一个关键的挑战仍然是阐明决定水氧化选择性的因素是通过所需的 4e-/4H+ 途径生成 O2 而不是通过 2e-/2H+ 生成 H2O2。我们现在表明,使用钴-钴 CoBr8 作为电催化剂进行水氧化,在均相溶液中提供 H2O2 作为主要产物,而由相同催化剂进行的非均相水氧化仅产生氧气。在该过程中形成的物种的实验和基于计算的研究揭示了 Co(III)-超氧化物中间体及其之前的高价 Co-oxyl 复合物的形成。确定了碱催化水解 Co(III)-氢过氧化物 [Co(III)-OOH]- 以释放 H2O2 与电化学氧化通过 [Co(III)-O2•]- 释放 O2 之间的竞争作为决定水氧化选择性的关键步骤。































 京公网安备 11010802027423号
京公网安备 11010802027423号