当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Polymorphism in Safinamide Acid Hydrochloride (Z′ = 3 and Z′ = 1) and Observation of a Temperature-Dependent Reversible Single-Crystal to Single-Crystal Phase Transformation of High-Z′ form (Z′ = 3 ↔ Z′ = 2 via an Intermediate Z′ = 4)
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.cgd.0c00958 Jagadeesh Babu Nanubolu 1, 2
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.cgd.0c00958 Jagadeesh Babu Nanubolu 1, 2
Affiliation
|
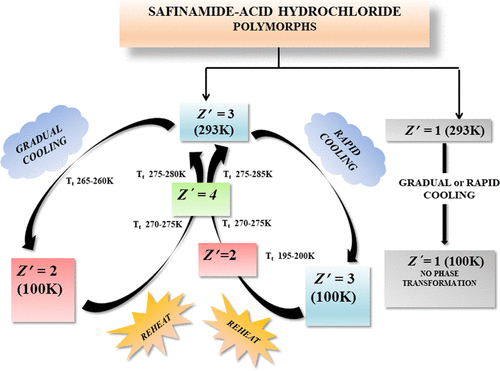
Two concomitant polymorphs of safinamide acid hydrochloride were obtained in an attempt to prepare the O-protonated amide salt of safinamide from ethanolic HCl solution. Polymorph I crystallized in the triclinic space group P1 with three molecules in the asymmetric unit (Z′ = 3) while polymorph II crystallized in the orthorhombic space group P212121 with a single molecule in the asymmetric unit (Z′ = 1). Structural differences at the conformational level and their influence on intermolecular interactions in the crystals led to the occurrence of polymorphism in the title compound. Both Z′ = 1 and Z′ = 3 polymorphic forms of safinamide acid hydrochloride were sustained by similar strong O–H···Cl– and N+–H···Cl– hydrogen-bonded interactions but differed significantly in the molecular conformations and hence their molecular arrangements in the crystal lattice. The C–H···O and C–H···F hydrogen bonds along with the strong hydrogen bonds facilitated one-dimensional supramolecular aggregates and caused the occurrence of the Z′ > 1 situation. The high-Z′ polymorph showed an interesting phase transition behavior during variable-temperature single-crystal X-ray diffraction studies (VT-SCXRD). When the sample was rapidly cooled from 293 K (room temperature) to 100 K (low temperature), the Z′ = 3 polymorph was stable; however, when the same crystal was subjected to gradual cooling from room temperature to 100 K, the Z′ = 3 polymorph transformed to a novel Z′ = 2 structure (in the transition temperature range 260−265 K). When the sample was warmed from 100 K to RT, the phase transition was found to be reversible from Z′ = 2 to Z′ = 3 (in the transition temperature range 270–275 K). Differential scanning calorimetry studies were conducted to confirm the reversible phase transition nature (Z′ = 3 ↔ Z′ = 2). An intermediate short-lived crystal form with four molecules in the asymmetric unit (Z′ = 4) was captured at 272 K during the VT-SCXRD warming cycle, which facilitated a better understanding of the structural reorganization process during the phase transition. The Z′ = 4 structure can be referred as a “crystal on the way” in the Z′ = 2 to Z′ = 3 phase transition. The close structural similarities among Z′ = 3, 2, and 4 accounted for the phase transformation in a single-crystal to single-crystal manner.
中文翻译:

构象多态性在沙芬酰胺盐酸盐(ž '= 3和ž '= 1)和一个与温度有关的可逆单晶观察到高的单结晶相变ž '形式(ž '= 3↔ ž '= 2通过中间Z '= 4)
试图从盐酸沙芬酰胺中得到两种伴随的多晶型物,以制备沙芬酰胺的O-质子化酰胺盐。多晶型物I在三斜空间群P 1中结晶,具有三个不对称单元(Z '= 3),而多晶型物II在正交晶空间群P 2 1 2 1 2 1中,具有单个分子在不对称单元(Z '中)结晶。= 1)。构象水平上的结构差异及其对晶体中分子间相互作用的影响导致标题化合物出现多态性。既ž '= 1和ž'沙非酰胺盐酸盐= 3种多晶型形式通过类似于强O - H···氯持续-和N + -H···氯-氢键合相互作用,但在分子构象,并因此在它们的分子安排显著不同水晶格子。C–H··O和C–H···F氢键与强氢键一起促进了一维超分子聚集,并导致Z '> 1的出现。高Z '多晶型物在可变温度单晶X射线衍射研究(VT-SCXRD)中显示出有趣的相变行为。当样品从293 K(室温)迅速冷却到100 K(低温)时,Z'= 3个多晶型物稳定;但是,当同一晶体从室温逐渐冷却到100 K时,Z '= 3的多晶型物转变为新颖的Z '= 2的结构(转变温度为260-265 K)。当样品从100 K加热到室温时,发现相变从Z '= 2到Z '= 3是可逆的(在270-275 K的转变温度范围内)。进行了差示扫描量的研究来证实可逆相变性质(ž '= 3↔ ž '= 2)。不对称单元中具有四个分子的中间短寿命晶体形式(Z′= 4)在VT-SCXRD升温周期的272 K时被捕获,这有助于更好地理解相变过程中的结构重组过程。所述ž '= 4的结构可以称为如在一个“在途中晶体” ž '= 2至ž '= 3相变。Z ′= 3、2和4之间的紧密结构相似性导致了单晶至单晶方式的相变。
更新日期:2021-01-06
中文翻译:

构象多态性在沙芬酰胺盐酸盐(ž '= 3和ž '= 1)和一个与温度有关的可逆单晶观察到高的单结晶相变ž '形式(ž '= 3↔ ž '= 2通过中间Z '= 4)
试图从盐酸沙芬酰胺中得到两种伴随的多晶型物,以制备沙芬酰胺的O-质子化酰胺盐。多晶型物I在三斜空间群P 1中结晶,具有三个不对称单元(Z '= 3),而多晶型物II在正交晶空间群P 2 1 2 1 2 1中,具有单个分子在不对称单元(Z '中)结晶。= 1)。构象水平上的结构差异及其对晶体中分子间相互作用的影响导致标题化合物出现多态性。既ž '= 1和ž'沙非酰胺盐酸盐= 3种多晶型形式通过类似于强O - H···氯持续-和N + -H···氯-氢键合相互作用,但在分子构象,并因此在它们的分子安排显著不同水晶格子。C–H··O和C–H···F氢键与强氢键一起促进了一维超分子聚集,并导致Z '> 1的出现。高Z '多晶型物在可变温度单晶X射线衍射研究(VT-SCXRD)中显示出有趣的相变行为。当样品从293 K(室温)迅速冷却到100 K(低温)时,Z'= 3个多晶型物稳定;但是,当同一晶体从室温逐渐冷却到100 K时,Z '= 3的多晶型物转变为新颖的Z '= 2的结构(转变温度为260-265 K)。当样品从100 K加热到室温时,发现相变从Z '= 2到Z '= 3是可逆的(在270-275 K的转变温度范围内)。进行了差示扫描量的研究来证实可逆相变性质(ž '= 3↔ ž '= 2)。不对称单元中具有四个分子的中间短寿命晶体形式(Z′= 4)在VT-SCXRD升温周期的272 K时被捕获,这有助于更好地理解相变过程中的结构重组过程。所述ž '= 4的结构可以称为如在一个“在途中晶体” ž '= 2至ž '= 3相变。Z ′= 3、2和4之间的紧密结构相似性导致了单晶至单晶方式的相变。































 京公网安备 11010802027423号
京公网安备 11010802027423号