Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2020-11-30 , DOI: 10.1016/j.csbj.2020.11.033 Jing Zhang 1, 2 , Yue Sun 2 , Li-Ye Zhong 2 , Nan-Nan Yu 2 , Lan Ouyang 2 , Run-Dong Fang 2 , Yang Wang 2 , Qing-Yu He 1, 2
|
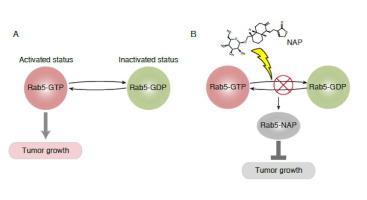
Rab5 is a small GTPase that plays a crucial role in oncogenic signal transduction, which was considered as an attractive target for cancer therapy. Rapid GDP/GTP exchange in the packet of Rab5 sustains its high activity for promoting cancer progression. However, Rab5 currently remains undruggable due to the lack of specific inhibitor. Herein, we reported the discovery of a novel Rab5 inhibitor, neoandrographolide (NAP), by using high-throughput virtual screening with a natural product library containing 7459 compounds, which can occupy the surface groove of Rab5, competing with GDP/GTP for the binding. Ser34 is the most important residue in the groove of Rab5, as it forms most hydrogen-bond interactions with GDP/GTP or NAP, and in silico mutation of Ser34 decreased the stabilization of Rab5. Moreover, fluorescence titration experiment and isothermal titration calorimetry (ITC) assay revealed a direct binding between NAP and Rab5. Biochemical and cell-based assays showed that NAP treatment not only diminished the activity of Rab5, but also suppressed cell growth of cancer cell. This finding firstly identifies NAP as a novel inhibitor of Rab5, which directly binds with Rab5 by occupying the GDP/GTP binding groove to suppress its functions, highlighting a great potential of NAP to be developed as a chemotherapeutic agent in cancer therapy.
中文翻译:

基于结构的新穿心莲内酯作为 Rab5 新型抑制剂抑制癌症生长的发现
Rab5 是一种小型 GTP 酶,在致癌信号转导中发挥着至关重要的作用,被认为是癌症治疗的一个有吸引力的靶标。 Rab5 包中的 GDP/GTP 快速交换维持了其促进癌症进展的高活性。然而,由于缺乏特异性抑制剂,Rab5 目前仍无法成药。在此,我们报道了一种新型Rab5抑制剂——新穿心莲内酯(NAP),通过对含有7459个化合物的天然产物库进行高通量虚拟筛选,它可以占据Rab5的表面凹槽,与GDP/GTP竞争结合。 Ser34 是 Rab5 凹槽中最重要的残基,因为它与 GDP/GTP 或 NAP 形成大部分氢键相互作用,并且 Ser34 的计算机突变降低了 Rab5 的稳定性。此外,荧光滴定实验和等温滴定量热法(ITC)测定揭示了NAP和Rab5之间的直接结合。生化和细胞检测表明,NAP 处理不仅降低了 Rab5 的活性,而且还抑制了癌细胞的生长。这一发现首次确定了NAP是一种新型的Rab5抑制剂,它通过占据GDP/GTP结合沟直接与Rab5结合,抑制其功能,凸显了NAP作为癌症治疗化疗药物的巨大潜力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号