Food Chemistry ( IF 8.5 ) Pub Date : 2020-11-28 , DOI: 10.1016/j.foodchem.2020.128728 Zhipeng Yu , Lixin Kang , Wenzhu Zhao , Sijia Wu , Long Ding , Fuping Zheng , Jingbo Liu , Jianrong Li
|
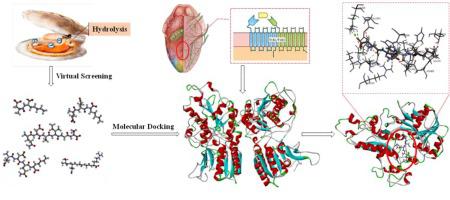
The structure of the umami receptor T1R1/T1R3 was constructed using homology modeling and molecular dynamics, and the interactions between peptides and this umami receptor were studied by molecular docking. The umami intensity of the peptides was also investigated by using an electronic tongue. The results showed that 99.3% of the amino acid residues in the homologous model of the T1R1/T1R3 heterodimer were within the allowable range, which is greater than the threshold requirement of 90% of the residues in the high-quality model structure. Five novel peptides (DK, EEK, EDQK, SEGGR, and QDSIGS) were selected and synthesized. The umami intensity of these five peptides was stronger than that of monosodium glutamate. The docking results revealed that the interactions between peptides and the major amino acids residues Arg151, Asp147, and Gln52 of T1R1 play critical roles in the production of umami taste.
中文翻译:

通过同源性建模和分子对接从肌球蛋白中鉴定新的鲜味肽
利用同源建模和分子动力学方法构建了鲜味受体T1R1 / T1R3的结构,并通过分子对接研究了肽与该鲜味受体之间的相互作用。还通过使用电子舌来研究了肽的鲜味强度。结果表明,T1R1 / T1R3异源二聚体同源模型中99.3%的氨基酸残基在允许范围内,大于高质量模型结构中90%残基的阈值要求。选择并合成了五种新型肽(DK,EEK,EDQK,SEGGR和QDSIGS)。这五个肽的鲜味强度比谷氨酸钠的鲜味强度强。对接结果表明,肽与主要氨基酸残基Arg151,Asp147,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号