当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Indolo[2,3-c]quinolin-6(7H)-ones and Antimalarial Isoneocryptolepine. Computational Study on the Pd-Catalyzed Intramolecular C–H Arylation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.joc.0c01832 Tímea Szabó 1 , Marcell Papp 1, 2 , Dóra Rita Németh 1 , András Dancsó 1 , Balázs Volk 1 , Mátyás Milen 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.joc.0c01832 Tímea Szabó 1 , Marcell Papp 1, 2 , Dóra Rita Németh 1 , András Dancsó 1 , Balázs Volk 1 , Mátyás Milen 1
Affiliation
|
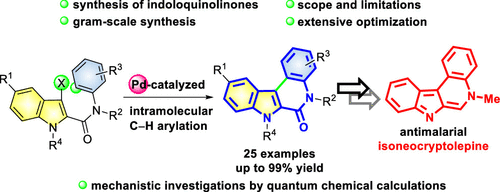
The synthesis of variously substituted indolo[2,3-c]quinolin-6(7H)-ones was developed via Pd-catalyzed intramolecular C–H arylation. This method highlights a strategy for preparing indoloquinoline precursors bearing versatile functional groups and provides a new approach for the synthesis of antimalarial isoneocryptolepine analogues. The plausible ring closure mechanism was examined with quantum chemical calculations, where a trigonal bipyramidal concerted metalation–deprotonation transition state is presumable.
中文翻译:

吲哚并[2,3 - c ]喹啉-6(7 H)-酮的合成及抗疟原虫烯隐油菜碱。Pd催化的分子内C–H芳基化的计算研究
通过Pd催化的分子内C–H芳基化反应,开发了各种取代的吲哚[2,3 - c ]喹啉-6(7 H)-ones的合成。该方法突出了制备带有多功能官能团的吲哚喹啉前体的策略,并为合成抗疟异异烯油菜碱类似物提供了新方法。通过量子化学计算研究了可能的闭环机理,推测是三角双锥体协同的金属化-去质子化过渡态。
更新日期:2021-01-01
中文翻译:

吲哚并[2,3 - c ]喹啉-6(7 H)-酮的合成及抗疟原虫烯隐油菜碱。Pd催化的分子内C–H芳基化的计算研究
通过Pd催化的分子内C–H芳基化反应,开发了各种取代的吲哚[2,3 - c ]喹啉-6(7 H)-ones的合成。该方法突出了制备带有多功能官能团的吲哚喹啉前体的策略,并为合成抗疟异异烯油菜碱类似物提供了新方法。通过量子化学计算研究了可能的闭环机理,推测是三角双锥体协同的金属化-去质子化过渡态。































 京公网安备 11010802027423号
京公网安备 11010802027423号