Science Bulletin ( IF 18.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.scib.2020.11.015
Yaqiong Guo 1, 2 , Ronghua Luo 3, 4, 5 , Yaqing Wang 1, 2 , Pengwei Deng 1, 2 , Tianzhang Song 3, 4, 5 , Min Zhang 1, 2 , Peng Wang 1 , Xu Zhang 1 , Kangli Cui 1, 2 , Tingting Tao 1, 2 , Zhongyu Li 1 , Wenwen Chen 1, 2 , Yongtang Zheng 4, 5, 6 , Jianhua Qin 1, 2, 6, 7
|
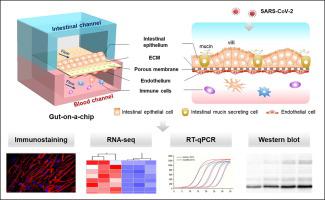
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global pandemic. Clinical evidence suggests that the intestine is another high-risk organ for SARS-CoV-2 infection besides the lungs. However, a model that can accurately reflect the response of the human intestine to the virus is still lacking. Here, we created an intestinal infection model on a chip that allows the recapitulation of human relevant intestinal pathophysiology induced by SARS-CoV-2 at organ level. This microengineered gut-on-chip reconstitutes the key features of the intestinal epithelium-vascular endothelium barrier through the three-dimensional (3D) co-culture of human intestinal epithelial, mucin-secreting, and vascular endothelial cells under physiological fluid flow. The intestinal epithelium showed permissiveness for viral infection and obvious morphological changes with injury of intestinal villi, dispersed distribution of mucus-secreting cells, and reduced expression of tight junction (E-cadherin), indicating the destruction of the intestinal barrier integrity caused by virus. Moreover, the vascular endothelium exhibited abnormal cell morphology, with disrupted adherent junctions. Transcriptional analysis revealed abnormal RNA and protein metabolism, as well as activated immune responses in both epithelial and endothelial cells after viral infection (e.g., upregulated cytokine genes), which may contribute to the injury of the intestinal barrier associated with gastrointestinal symptoms. This human organ system can partially mirror intestinal barrier injury and the human response to viral infection, which is not possible in existing in vitro culture models. It provides a unique and rapid platform to accelerate COVID-19 research and develop novel therapies.
中文翻译:

SARS-CoV-2通过仿生人体肠道芯片诱导肠道反应
由严重急性呼吸系统综合症冠状病毒2(SARS-CoV-2)引起的冠状病毒病2019(COVID-19)已成为全球性大流行。临床证据表明,除了肺部以外,肠道是SARS-CoV-2感染的另一高危器官。但是,仍然缺乏能够准确反映人肠道对病毒的反应的模型。在这里,我们在芯片上创建了一个肠道感染模型,该模型可以在器官水平上概括由SARS-CoV-2诱导的人类相关的肠道病理生理。这种微工程芯片上肠道通过在生理性流体流下人肠上皮,粘液分泌和血管内皮细胞的三维(3D)共培养,重构了肠上皮-血管内皮屏障的关键特征。肠道上皮表现出对病毒感染的许可性,并且随着肠绒毛的损伤,粘液分泌细胞的分散分布以及紧密连接(E-钙粘蛋白)的表达减少而出现明显的形态变化,表明病毒引起的肠屏障完整性的破坏。此外,血管内皮表现出异常的细胞形态,具有破坏的粘附连接。转录分析显示病毒感染后上皮细胞和内皮细胞中异常的RNA和蛋白质代谢以及激活的免疫反应(例如,上调的细胞因子基因),这可能导致与胃肠道症状相关的肠屏障损伤。这种人体器官系统可以部分反映肠道屏障损伤和人体对病毒感染的反应,体外培养模型。它提供了一个独特而快速的平台来加速COVID-19的研究和开发新疗法。







































 京公网安备 11010802027423号
京公网安备 11010802027423号