当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular Synthesis of Organoboron Helically Chiral Compounds: Cutouts from Extended Helices
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-26 , DOI: 10.1002/anie.202014138 Julian Full 1, 2 , Santosh P Panchal 1, 2 , Julian Götz 2 , Ana-Maria Krause 2 , Agnieszka Nowak-Król 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-26 , DOI: 10.1002/anie.202014138 Julian Full 1, 2 , Santosh P Panchal 1, 2 , Julian Götz 2 , Ana-Maria Krause 2 , Agnieszka Nowak-Król 1, 2
Affiliation
|
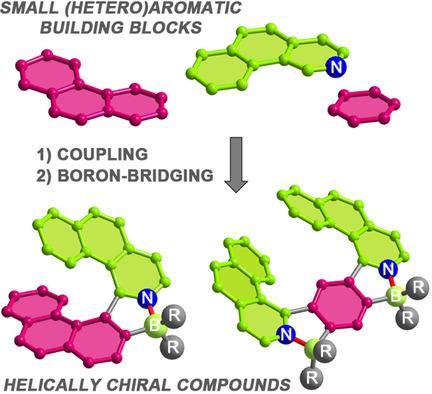
Two types of helically chiral compounds bearing one and two boron atoms were synthesized by a modular approach. Formation of the helical scaffolds was executed by the introduction of boron to flexible biaryl and triaryl derived from small achiral building blocks. All‐ortho‐fused azabora[7]helicenes feature exceptional configurational stability, blue or green fluorescence with quantum yields (Φfl) of 18–24 % in solution, green or yellow solid‐state emission (Φfl up to 23 %), and strong chiroptical response with large dissymmetry factors of up to 1.12×10−2. Azabora[9]helicenes consisting of angularly and linearly fused rings are blue emitters exhibiting Φfl of up to 47 % in CH2Cl2 and 25 % in the solid state. As revealed by the DFT calculations, their P–M interconversion pathway is more complex than that of H1. Single‐crystal X‐ray analysis shows clear differences in the packing arrangement of methyl and phenyl derivatives. These molecules are proposed as primary structures of extended helices.
中文翻译:

有机硼螺旋手性化合物的模块化合成:延伸螺旋的切口
通过模块化方法合成了两种带有一个和两个硼原子的螺旋手性化合物。通过将硼引入源自小的非手性结构单元的柔性联芳基和三芳基来形成螺旋支架。全邻位稠合azabora[7]螺旋烯具有优异的构型稳定性,溶液中量子产率(Φfl )为18-24%的蓝色或绿色荧光,绿色或黄色固态发射(Φfl高达23%),以及具有高达1.12×10 -2的大不对称因子的强手性光学响应。由成角度和线性稠合的环组成的Azabora[9]螺旋烯是蓝色发射体,其在CH 2 Cl 2中的Φ fl高达47% ,在固态下高达25%。DFT 计算表明,它们的 P-M 互变途径比H1更复杂。单晶 X 射线分析显示甲基和苯基衍生物的堆积排列存在明显差异。这些分子被认为是延伸螺旋的一级结构。
更新日期:2020-11-26
中文翻译:

有机硼螺旋手性化合物的模块化合成:延伸螺旋的切口
通过模块化方法合成了两种带有一个和两个硼原子的螺旋手性化合物。通过将硼引入源自小的非手性结构单元的柔性联芳基和三芳基来形成螺旋支架。全邻位稠合azabora[7]螺旋烯具有优异的构型稳定性,溶液中量子产率(Φfl )为18-24%的蓝色或绿色荧光,绿色或黄色固态发射(Φfl高达23%),以及具有高达1.12×10 -2的大不对称因子的强手性光学响应。由成角度和线性稠合的环组成的Azabora[9]螺旋烯是蓝色发射体,其在CH 2 Cl 2中的Φ fl高达47% ,在固态下高达25%。DFT 计算表明,它们的 P-M 互变途径比H1更复杂。单晶 X 射线分析显示甲基和苯基衍生物的堆积排列存在明显差异。这些分子被认为是延伸螺旋的一级结构。































 京公网安备 11010802027423号
京公网安备 11010802027423号