Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The molecular insight into the “Zeolite-ice” as hydrogen storage material
Energy ( IF 9.0 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.energy.2020.119406 Yanhong Wang , Kaidong Yin , Shuanshi Fan , Xuemei Lang , Chi Yu , Shenglong Wang , Song Li
Energy ( IF 9.0 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.energy.2020.119406 Yanhong Wang , Kaidong Yin , Shuanshi Fan , Xuemei Lang , Chi Yu , Shenglong Wang , Song Li
|
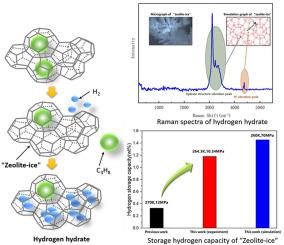
Abstract “Zeolite-ice” is cavities structure constructed by water molecules that held together by hydrogen-bonds, formed by propane remove from propane hydrate, which structure is similar to zeolite. Hydrogen stored in “Zeolite-ice” is a novel method, which was investigated through experiments and molecular simulations in this paper. Hydrogen storage capacity and the hydrate structures were characterized by Raman spectra. To provide insight into experiments, a series MD simulation had been performed under 250 K–270 K and 30–70 MPa. The results showed that pressure and temperature could influence hydrogen storage performance of propane hydrate. At 270 K, the amount of hydrogen molecules that entered hydrate phase increased with increasing pressure, while hydrogen storage capacity merely fluctuated around 1.0 wt%-1.5 wt% at 260 K and 250 K. Besides, under 60 and 70 MPa, diffusion coefficient of hydrogen molecules at 260 K became relatively low, which led to higher hydrogen capacity than that at 250 K. F4 order parameter analysis and free-energy surface proved that the temperature impacted the stability of cavities of hydrate. This stability caused different motion of hydrogen molecules that doubly occupying large cages. It was feasible to use propane hydrate as a hydrogen storage material and control the pressure and temperature to develop its potential of hydrogen storage.
中文翻译:

“沸石-冰”作为储氢材料的分子洞察
摘要 “沸石-冰”是由水分子通过氢键结合在一起构成的空腔结构,由丙烷从丙烷水合物中脱出而形成,其结构类似于沸石。在“沸石-冰”中储存氢气是一种新方法,本文通过实验和分子模拟对其进行了研究。储氢能力和水合物结构通过拉曼光谱表征。为了深入了解实验,在 250 K-270 K 和 30-70 MPa 下进行了一系列 MD 模拟。结果表明,压力和温度会影响丙烷水合物的储氢性能。在 270 K 时,进入水合物相的氢分子数量随着压力的增加而增加,而储氢能力在 260 K 和 250 K 时仅波动 1.0 wt%-1.5 wt% 左右。此外,在 60 和 70 MPa 下,氢分子在 260 K 的扩散系数相对较低,导致氢容量高于 250 K。 F4 阶参数分析和自由能表面证明,温度影响水合物腔的稳定性. 这种稳定性导致双占据大笼子的氢分子的不同运动。以丙烷水合物作为储氢材料,通过控制压力和温度来发挥其储氢潜力是可行的。这种稳定性导致双占据大笼子的氢分子的不同运动。以丙烷水合物作为储氢材料,通过控制压力和温度来发挥其储氢潜力是可行的。这种稳定性导致双占据大笼子的氢分子的不同运动。以丙烷水合物作为储氢材料,通过控制压力和温度来发挥其储氢潜力是可行的。
更新日期:2021-02-01
中文翻译:

“沸石-冰”作为储氢材料的分子洞察
摘要 “沸石-冰”是由水分子通过氢键结合在一起构成的空腔结构,由丙烷从丙烷水合物中脱出而形成,其结构类似于沸石。在“沸石-冰”中储存氢气是一种新方法,本文通过实验和分子模拟对其进行了研究。储氢能力和水合物结构通过拉曼光谱表征。为了深入了解实验,在 250 K-270 K 和 30-70 MPa 下进行了一系列 MD 模拟。结果表明,压力和温度会影响丙烷水合物的储氢性能。在 270 K 时,进入水合物相的氢分子数量随着压力的增加而增加,而储氢能力在 260 K 和 250 K 时仅波动 1.0 wt%-1.5 wt% 左右。此外,在 60 和 70 MPa 下,氢分子在 260 K 的扩散系数相对较低,导致氢容量高于 250 K。 F4 阶参数分析和自由能表面证明,温度影响水合物腔的稳定性. 这种稳定性导致双占据大笼子的氢分子的不同运动。以丙烷水合物作为储氢材料,通过控制压力和温度来发挥其储氢潜力是可行的。这种稳定性导致双占据大笼子的氢分子的不同运动。以丙烷水合物作为储氢材料,通过控制压力和温度来发挥其储氢潜力是可行的。这种稳定性导致双占据大笼子的氢分子的不同运动。以丙烷水合物作为储氢材料,通过控制压力和温度来发挥其储氢潜力是可行的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号