当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium Pybox-Catalyzed Enantioselective Intramolecular C–H Amination of Sulfamoyl Azides en Route to Chiral Vicinal Diamines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.joc.0c02279 Xin Nie 1 , Zihan Yan 1 , Sergei Ivlev 1 , Eric Meggers 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.joc.0c02279 Xin Nie 1 , Zihan Yan 1 , Sergei Ivlev 1 , Eric Meggers 1
Affiliation
|
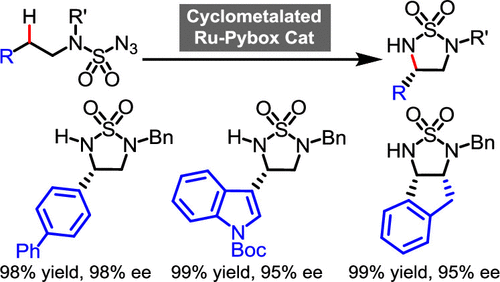
Enantioselective C(sp3)–H aminations allow an efficient access to nonracemic chiral amines. This work reports the catalytic asymmetric synthesis of chiral 1,2,5-thiadiazolidine-1,1-dioxides by an enantioselective ring-closing 1,5-C–H amination of sulfamoyl azides. The reaction is catalyzed by a recently introduced simple chiral ruthenium bis(oxazoline) (pybox) complex ( Angew. Chem. Int. Ed. 2020, 59, 12395) and provides cyclic 5-membered sulfamide products in up to 98% yield and up to 98% ee if the C–H bond is in a benzylic position. Mechanistic experiments support a stepwise mechanism in which an intermediate ruthenium nitrenoid species initiates a 1,5-hydrogen atom transfer followed by an immediate radical rebound. The cyclic sulfamide products are suitable intermediates for the synthesis of chiral vicinal diamines as has been verified for a representative example.
中文翻译:

钌磺胺催化的氨磺酰叠氮化物在手性邻二胺中的对映选择性分子内CH反应
对映选择性的C(sp 3)–H胺可有效地获得非外消旋手性胺。这项工作报告了通过氨磺酰氨基叠氮化物的对映选择性闭环1,5-CH胺催化的手性1,2,5-噻二唑烷-1,1-二氧化物的催化不对称合成。该反应通过一个最近推出的简单的手性钌双(恶唑啉)(pybox)配合物(催化的Angew。化学式中间体版 2020,59(12395)并以高达98%的收率和高达98%ee的价格提供环状5元磺酰胺产物(如果C–H键位于苄基位置)。力学实验支持逐步机制,其中中间的钌亚硝烯类物质引发1,5-氢原子转移,然后立即发生自由基反弹。环状磺酰胺产物是用于合成手性邻二胺的合适中间体,已为代表性实例证实。
更新日期:2021-01-01
中文翻译:

钌磺胺催化的氨磺酰叠氮化物在手性邻二胺中的对映选择性分子内CH反应
对映选择性的C(sp 3)–H胺可有效地获得非外消旋手性胺。这项工作报告了通过氨磺酰氨基叠氮化物的对映选择性闭环1,5-CH胺催化的手性1,2,5-噻二唑烷-1,1-二氧化物的催化不对称合成。该反应通过一个最近推出的简单的手性钌双(恶唑啉)(pybox)配合物(催化的Angew。化学式中间体版 2020,59(12395)并以高达98%的收率和高达98%ee的价格提供环状5元磺酰胺产物(如果C–H键位于苄基位置)。力学实验支持逐步机制,其中中间的钌亚硝烯类物质引发1,5-氢原子转移,然后立即发生自由基反弹。环状磺酰胺产物是用于合成手性邻二胺的合适中间体,已为代表性实例证实。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号