Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 4.6 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.bbamcr.2020.118913 Magdalena Gryzik , Michela Asperti , Andrea Denardo , Paolo Arosio , Maura Poli
|
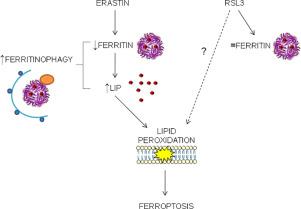
Ferroptosis is a regulated cell death characterized by a lethal accumulation of lipid peroxides due to an increase of intracellular iron and a decrease of antioxidant capacity. The reduction of antioxidant activity is obtained by using chemical agents, such as erastin and RSL3, the first one inhibiting the transmembrane cystine-glutamate antiporter causing a cysteine and glutathione depletion and the second one inactivating directly the glutathione peroxidase 4 (GPX4) respectively. The role of iron and its related proteins in supporting the formation of lipid peroxides, is not completely understood hence to try to shed light on it we generated HeLa clones with altered ferritinophagy, the ferritin degradation process, by knocking-out or overexpressing Nuclear Receptor Coactivator 4 (NCOA4), the ferritin autophagic cargo-receptor. NCOA4 deficiency abolished ferritinophagy increasing ferritin level and making the cells more resistant to erastin, but unexpectedly more sensitive to RSL3. Interestingly, we found that erastin promoted ferritinophagy in HeLa cells expressing NCOA4, increasing the free iron, lipid peroxidation and the sensitivity to ferroptosis. In contrast, RSL3 did not modulate ferritinophagy, while NCOA4 overexpression delayed RSL3-induced cell death suggesting that RSL3 mechanism of action is independent of ferritin degradation process. Therefore, the ferritin-iron release in the execution of ferroptosis seems to depend on the inducing compound, its target and downstream pathway of cell death activation.
中文翻译:

NCOA4介导的铁蛋白吞噬作用促进Hestin细胞中由蛋白蛋白引起的肥大症,但不引起RSL3
Ferroptosis是受调节的细胞死亡,其特征是由于细胞内铁的增加和抗氧化剂能力的降低,脂质过氧化物的致死性积累。抗氧化剂活性的降低是通过使用化学试剂(例如erastin和RSL3)来实现的,第一种抑制跨膜胱氨酸-谷氨酸反转运蛋白导致半胱氨酸和谷胱甘肽耗竭,第二种分别直接使谷胱甘肽过氧化物酶4(GPX4)失活。铁及其相关蛋白在支持脂质过氧化物形成中的作用尚不完全清楚,因此,为了阐明这一点,我们通过敲除或过表达核受体共激活剂,生成了铁蛋白降解,铁蛋白降解过程改变的HeLa克隆。 4(NCOA4),铁蛋白自噬货物受体。NCOA4缺乏症消除了铁蛋白吞噬作用,从而增加了铁蛋白水平,并使细胞对蛋白的抵抗力增强,但出乎意料地对RSL3更加敏感。有趣的是,我们发现时代蛋白在表达NCOA4的HeLa细胞中促进铁蛋白吞噬,从而增加了游离铁,脂质过氧化作用以及对肥大病的敏感性。相反,RSL3不能调节铁蛋白吞噬,而NCOA4的过表达延迟RSL3诱导的细胞死亡,这表明RSL3的作用机理与铁蛋白降解过程无关。因此,在进行肥大病的过程中铁蛋白-铁的释放似乎取决于诱导化合物,其靶标和细胞死亡激活的下游途径。我们发现,Eaststin促进表达NCOA4的HeLa细胞中的铁蛋白吞噬作用,从而增加了游离铁,脂质过氧化作用以及对肥大症的敏感性。相反,RSL3不能调节铁蛋白吞噬,而NCOA4的过表达延迟RSL3诱导的细胞死亡,这表明RSL3的作用机制与铁蛋白降解过程无关。因此,在进行肥大病的过程中铁蛋白-铁的释放似乎取决于诱导化合物,其靶标和细胞死亡激活的下游途径。我们发现,Eaststin促进表达NCOA4的HeLa细胞中的铁蛋白吞噬作用,从而增加了游离铁,脂质过氧化作用以及对肥大症的敏感性。相反,RSL3不能调节铁蛋白吞噬,而NCOA4的过表达延迟RSL3诱导的细胞死亡,这表明RSL3的作用机制与铁蛋白降解过程无关。因此,在进行肥大病的过程中铁蛋白-铁的释放似乎取决于诱导化合物,其靶标和细胞死亡激活的下游途径。而NCOA4的过表达会延迟RSL3诱导的细胞死亡,这表明RSL3的作用机制与铁蛋白降解过程无关。因此,在进行肥大病的过程中铁蛋白-铁的释放似乎取决于诱导化合物,其靶标和细胞死亡激活的下游途径。而NCOA4的过表达会延迟RSL3诱导的细胞死亡,这表明RSL3的作用机制与铁蛋白降解过程无关。因此,在进行肥大病的过程中铁蛋白-铁的释放似乎取决于诱导化合物,其靶标和细胞死亡激活的下游途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号