Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Function of N-Acetylmannosamine Kinases from Pathogenic Bacteria
ACS Omega ( IF 3.7 ) Pub Date : 2020-11-23 , DOI: 10.1021/acsomega.0c03699 Thanuja Gangi Setty 1, 2 , Arunabha Sarkar 3 , David Coombes 4 , Renwick C. J. Dobson 4, 5 , Ramaswamy Subramanian 1, 6
ACS Omega ( IF 3.7 ) Pub Date : 2020-11-23 , DOI: 10.1021/acsomega.0c03699 Thanuja Gangi Setty 1, 2 , Arunabha Sarkar 3 , David Coombes 4 , Renwick C. J. Dobson 4, 5 , Ramaswamy Subramanian 1, 6
Affiliation
|
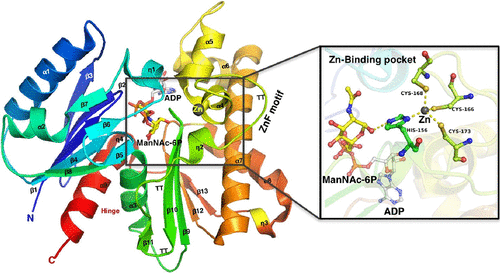
Several pathogenic bacteria import and catabolize sialic acids as a source of carbon and nitrogen. Within the sialic acid catabolic pathway, the enzyme N-acetylmannosamine kinase (NanK) catalyzes the phosphorylation of N-acetylmannosamine to N-acetylmannosamine-6-phosphate. This kinase belongs to the ROK superfamily of enzymes, which generally contain a conserved zinc-finger (ZnF) motif that is important for their structure and function. Previous structural studies have shown that the ZnF motif is absent in NanK of Fusobacterium nucleatum (Fn-NanK), a Gram-negative bacterium that causes the gum disease gingivitis. However, the effect in loss of the ZnF motif on the kinase activity is unknown. Using kinetic and thermodynamic studies, we have studied the functional properties of Fn-NanK to its substrates ManNAc and ATP, compared its activity with other ZnF motif-containing NanK enzymes from closely related Gram-negative pathogenic bacteria Haemophilus influenzae (Hi-NanK), Pasteurella multocida (Pm-NanK), and Vibrio cholerae (Vc-NanK). Our studies show a 10-fold decrease in substrate binding affinity between Fn-NanK (apparent KM ≈ 700 μM) and ZnF motif-containing NanKs (apparent KM ≈ 60 μM). To understand the structural features that combat the loss of the ZnF motif in Fn-NanK, we solved the crystal structures of functionally homologous ZnF motif-containing NanKs from P. multocida and H. influenzae. Here, we report Pm-NanK:unliganded, Pm-NanK:AMPPNP, Pm-NanK:ManNAc, Hi-NanK:ManNAc, and Hi-NanK:ManNAc-6P:ADP crystal structures. Structural comparisons of Fn-NanK with Hi-NanK, Pm-NanK, and hMNK (human N-acetylmannosamine kinase domain of UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase, GNE) show that even though there is less sequence identity, they have high degree of structural similarity. Furthermore, our structural analyses highlight that the ZnF motif of Fn-NanK is substituted by a set of hydrophobic residues, which forms a hydrophobic cluster that helps the proper orientation of ManNac in the active site. In summary, ZnF-containing and ZnF-lacking NanK enzymes from different Gram-negative pathogenic bacteria are functionally very similar but differ in their metal requirement. Our structural studies unveil the structural modifications in Fn-NanK that compensate the loss of the ZnF motif in comparison to other NanK enzymes.
中文翻译:

病原细菌N-乙酰甘露糖胺激酶的结构和功能
几种致病细菌通过唾液酸进口和分解代谢唾液酸作为碳和氮的来源。在唾液酸分解代谢途径中,酶N-乙酰甘露糖胺激酶(NanK)催化N-乙酰甘露糖胺磷酸化为N-乙酰甘露糖胺6-磷酸。该激酶属于酶的ROK超家族,通常含有保守的锌指(ZnF)基序,这对于它们的结构和功能很重要。先前的结构研究表明,在Fusobacter nucleatum(Fn)的NanK中不存在ZnF基序-NanK),一种引起牙龈疾病齿龈炎的革兰氏阴性细菌。然而,ZnF基序丧失对激酶活性的影响尚不清楚。通过动力学和热力学研究,我们研究了Fn- NanK对其底物ManNAc和ATP的功能特性,并将其与来自密切相关的革兰氏阴性致病菌流感嗜血杆菌(Hi- NanK)的其他含ZnF主题的NanK酶进行了比较,多杀巴斯德氏菌(Pm -NanK)和霍乱弧菌(Vc -NanK)。我们的研究表明Fn- NanK(表观K M≈700μM)和含有的ZnF基序的NanKs(表观ķ中号≈60μM)。为了了解对抗Fn- NanK中ZnF基序丢失的结构特征,我们解决了多杀性疟原虫和流感嗜血杆菌中含有功能性同源ZnF基序的NanKs的晶体结构。在这里,我们报告Pm -NanK:未配体,Pm -NanK:AMPPNP,Pm -NanK:ManNAc,Hi -NanK:ManNAc和Hi -NanK:ManNAc-6P:ADP晶体结构。的结构比较FN -NanK与您好-NanK,PM -NanK,和hMNK(人ÑUDP- N-乙酰葡糖胺-2-表异构酶/ N-乙酰甘露糖胺激酶(GNE)的β-乙酰甘露糖胺激酶结构域显示,即使序列同一性较低,它们也具有高度的结构相似性。此外,我们的结构分析突出表明Fn- NanK的ZnF基序被一组疏水残基取代,后者形成疏水簇,有助于ManNac在活性位点中正确定位。总之,来自不同革兰氏阴性致病菌的含ZnF和缺少ZnF的NanK酶在功能上非常相似,但金属需求不同。我们的结构研究揭示了Fn中的结构修改与其他NanK酶相比,可补偿ZnF基序丧失的-NanK。
更新日期:2020-12-08
中文翻译:

病原细菌N-乙酰甘露糖胺激酶的结构和功能
几种致病细菌通过唾液酸进口和分解代谢唾液酸作为碳和氮的来源。在唾液酸分解代谢途径中,酶N-乙酰甘露糖胺激酶(NanK)催化N-乙酰甘露糖胺磷酸化为N-乙酰甘露糖胺6-磷酸。该激酶属于酶的ROK超家族,通常含有保守的锌指(ZnF)基序,这对于它们的结构和功能很重要。先前的结构研究表明,在Fusobacter nucleatum(Fn)的NanK中不存在ZnF基序-NanK),一种引起牙龈疾病齿龈炎的革兰氏阴性细菌。然而,ZnF基序丧失对激酶活性的影响尚不清楚。通过动力学和热力学研究,我们研究了Fn- NanK对其底物ManNAc和ATP的功能特性,并将其与来自密切相关的革兰氏阴性致病菌流感嗜血杆菌(Hi- NanK)的其他含ZnF主题的NanK酶进行了比较,多杀巴斯德氏菌(Pm -NanK)和霍乱弧菌(Vc -NanK)。我们的研究表明Fn- NanK(表观K M≈700μM)和含有的ZnF基序的NanKs(表观ķ中号≈60μM)。为了了解对抗Fn- NanK中ZnF基序丢失的结构特征,我们解决了多杀性疟原虫和流感嗜血杆菌中含有功能性同源ZnF基序的NanKs的晶体结构。在这里,我们报告Pm -NanK:未配体,Pm -NanK:AMPPNP,Pm -NanK:ManNAc,Hi -NanK:ManNAc和Hi -NanK:ManNAc-6P:ADP晶体结构。的结构比较FN -NanK与您好-NanK,PM -NanK,和hMNK(人ÑUDP- N-乙酰葡糖胺-2-表异构酶/ N-乙酰甘露糖胺激酶(GNE)的β-乙酰甘露糖胺激酶结构域显示,即使序列同一性较低,它们也具有高度的结构相似性。此外,我们的结构分析突出表明Fn- NanK的ZnF基序被一组疏水残基取代,后者形成疏水簇,有助于ManNac在活性位点中正确定位。总之,来自不同革兰氏阴性致病菌的含ZnF和缺少ZnF的NanK酶在功能上非常相似,但金属需求不同。我们的结构研究揭示了Fn中的结构修改与其他NanK酶相比,可补偿ZnF基序丧失的-NanK。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号