当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Photo-oxidation of Methane to Methanol with Oxygen over Dual-Cocatalyst-Modified Titanium Dioxide
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-23 , DOI: 10.1021/acscatal.0c04329 Hui Song 1, 2 , Xianguang Meng 1, 3 , Shengyao Wang 1, 4 , Wei Zhou 5 , Shuang Song 1 , Tetsuya Kako 1 , Jinhua Ye 1, 2, 6
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-23 , DOI: 10.1021/acscatal.0c04329 Hui Song 1, 2 , Xianguang Meng 1, 3 , Shengyao Wang 1, 4 , Wei Zhou 5 , Shuang Song 1 , Tetsuya Kako 1 , Jinhua Ye 1, 2, 6
Affiliation
|
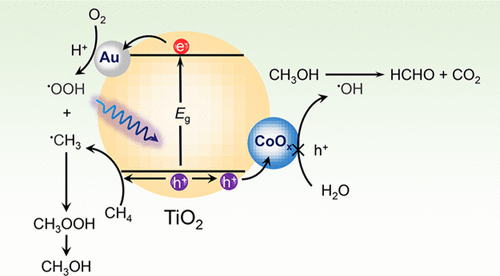
Direct and selective oxidation of CH4 with dioxygen to methanol is a “dream reaction” in modern catalysis yet remains a great challenge. Here, we report that TiO2 loaded with dual cocatalysts, that is, nanometals and cobalt oxide (CoOx) nanoclusters, is capable of selectively catalyzing CH4 to CH3OH at room temperature under photoexcitation using abundant and inexpensive O2 as an oxidant. The best activity for the formation of the primary products, CH3OOH and CH3OH, is up to 50.8 μmol for 2 h with 95% selectivity. Mechanistic studies elucidate that the superior activity and selectivity result from the synergistic effect of nanometals and CoOx. Nanometals enhance CH4 conversion by promoting the separation of the photoexcited electron and the reduction of O2. The CoOx mediates a mild CH4 oxidation process by suppressing the formation of highly oxidative •OH radicals that can further oxidize CH3OH to HCHO and CO2, thereby preserving a high selectivity toward oxygenated products. This work provides a prototype in designing efficient photocatalysts for selective oxidation of CH4 to CH3OH with O2 under mild conditions.
中文翻译:

在双助催化剂修饰的二氧化钛上用氧气将甲烷选择性光氧化为甲醇
在现代催化中,用双氧将CH 4直接选择性氧化为甲醇是一个“梦想反应”,但仍然是一个巨大的挑战。在此,我们报道了负载有双重助催化剂(即纳米金属和氧化钴(CoO x)纳米团簇)的TiO 2能够在室温下在光激发下使用大量廉价的O 2作为氧化剂选择性催化CH 4至CH 3 OH。。形成初级产物CH 3 OOH和CH 3的最佳活性OH在2小时内达到50.8μmol,选择性为95%。机理研究表明,优异的活性和选择性是由纳米金属和CoO x的协同作用产生的。纳米金属通过促进光激发电子的分离和O 2的还原来增强CH 4的转化。CoO x通过抑制可进一步将CH 3 OH氧化为HCHO和CO 2的高度氧化• OH自由基的形成,介导了温和的CH 4氧化过程,从而保持了对氧化产物的高选择性。这项工作为设计用于CH 4选择性氧化的有效光催化剂提供了一个原型。在温和条件下用O 2转化为CH 3 OH 。
更新日期:2020-12-04
中文翻译:

在双助催化剂修饰的二氧化钛上用氧气将甲烷选择性光氧化为甲醇
在现代催化中,用双氧将CH 4直接选择性氧化为甲醇是一个“梦想反应”,但仍然是一个巨大的挑战。在此,我们报道了负载有双重助催化剂(即纳米金属和氧化钴(CoO x)纳米团簇)的TiO 2能够在室温下在光激发下使用大量廉价的O 2作为氧化剂选择性催化CH 4至CH 3 OH。。形成初级产物CH 3 OOH和CH 3的最佳活性OH在2小时内达到50.8μmol,选择性为95%。机理研究表明,优异的活性和选择性是由纳米金属和CoO x的协同作用产生的。纳米金属通过促进光激发电子的分离和O 2的还原来增强CH 4的转化。CoO x通过抑制可进一步将CH 3 OH氧化为HCHO和CO 2的高度氧化• OH自由基的形成,介导了温和的CH 4氧化过程,从而保持了对氧化产物的高选择性。这项工作为设计用于CH 4选择性氧化的有效光催化剂提供了一个原型。在温和条件下用O 2转化为CH 3 OH 。































 京公网安备 11010802027423号
京公网安备 11010802027423号