当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small-Molecule Inhibitors Directly Targeting KRAS as Anticancer Therapeutics
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jmedchem.0c01312 Hao Chen 1 , Jeff B. Smaill 2 , Tongzheng Liu 1 , Ke Ding 1 , Xiaoyun Lu 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jmedchem.0c01312 Hao Chen 1 , Jeff B. Smaill 2 , Tongzheng Liu 1 , Ke Ding 1 , Xiaoyun Lu 1
Affiliation
|
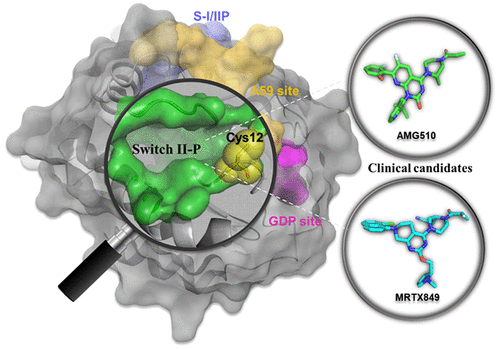
KRAS, the most frequently mutated oncogene, plays a predominant role in driving initiation and progression of cancers. Decades of effort to target KRAS using small molecules has been unsuccessful, causing KRAS to be considered an “undruggable” cancer target. However, this view began to change recently, as drug discovery techniques have developed several KRAS G12C allosteric inhibitors that are currently being evaluated in clinical trials. Herein we provide an in-depth analysis of the structure and binding pockets of KRAS, medicinal chemistry optimization processes, and the biological characterization of small-molecule inhibitors that directly target KRAS, including covalent allosteric inhibitors specific for the G12C mutant, GTP-competitive inhibitors targeting the nucleotide-binding site, and protein–protein interaction inhibitors that bind in the switch I/II pocket or the A59 site. Additionally, we propose potential challenges faced by these new classes of KRAS inhibitors under clinical evaluation.
中文翻译:

小分子抑制剂直接靶向KRAS作为抗癌药物
KRAS是最常见的致癌基因,在驱动癌症的发生和发展中起主要作用。使用小分子靶向KRAS的数十年努力一直未成功,导致KRAS被认为是“不可治疗的”癌症靶标。但是,随着药物发现技术开发出几种KRAS G12C变构抑制剂,这种观点最近开始改变,目前正在临床试验中对其进行评估。本文中,我们对KRAS的结构和结合口袋,药物化学优化过程以及直接靶向KRAS的小分子抑制剂(包括特异于G12C突变体的共价变构抑制剂,GTP竞争性抑制剂)的生物学特性进行了深入分析。靶向核苷酸结合位点,以及在开关I / II口袋或A59位点结合的蛋白质-蛋白质相互作用抑制剂。此外,我们提出了在临床评估中这些新型KRAS抑制剂面临的潜在挑战。
更新日期:2020-12-10
中文翻译:

小分子抑制剂直接靶向KRAS作为抗癌药物
KRAS是最常见的致癌基因,在驱动癌症的发生和发展中起主要作用。使用小分子靶向KRAS的数十年努力一直未成功,导致KRAS被认为是“不可治疗的”癌症靶标。但是,随着药物发现技术开发出几种KRAS G12C变构抑制剂,这种观点最近开始改变,目前正在临床试验中对其进行评估。本文中,我们对KRAS的结构和结合口袋,药物化学优化过程以及直接靶向KRAS的小分子抑制剂(包括特异于G12C突变体的共价变构抑制剂,GTP竞争性抑制剂)的生物学特性进行了深入分析。靶向核苷酸结合位点,以及在开关I / II口袋或A59位点结合的蛋白质-蛋白质相互作用抑制剂。此外,我们提出了在临床评估中这些新型KRAS抑制剂面临的潜在挑战。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号