当前位置:
X-MOL 学术
›
Appl. Catal. A Gen.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into CHx formation in Fischer–Tropsch synthesis on the hexahedron Co catalyst: Effect of surface structure on the preferential mechanism and existence form
Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2016-07-22 13:46:40
Riguang Zhang, Fu Liu, Qiang Wang, Baojun Wang, Debao Li
Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2016-07-22 13:46:40
Riguang Zhang, Fu Liu, Qiang Wang, Baojun Wang, Debao Li
|
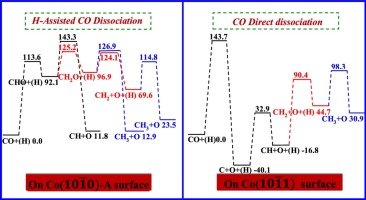
Spin-polarized density functional theory calculations have been performed to investigate the preferential mechanism of CH x (x =1–3) formation in Fischer-Tropsch synthesis on only the hexahedron Co( 10 1 ¯ 0 )-A and Co( 10 1 ¯ 0 ) surfaces. Our results show that CO hydrogenation to CHO is favored compared to CO direct dissociation and hydrogenation to COH on these two surfaces. Starting from C and CHO, we further seek out the optimal pathways of CH x formation, suggesting that CH x is mainly formed through H-assisted CO dissociation pathways on Co( 10 1 ¯ 0 )-A surface, in which CH is form via CHO dissociation, CH2 and CH3 are formed through CH2O with the direct and H-assisted dissociation, respectively; meanwhile, CH2 hydrogenation also contributes to CH3 formation; CH2 and CH3 are the surface abundant species on Co( 10 1 ¯ 0 )-A surface. However, on Co( 10 1 ¯ 1 ) surface, CH x species is formed through CO direct dissociation into C, followed by C successive hydrogenation, C and CH are the surface abundant species. Therefore, Co surface structure can affect the preferential formation pathways and the dominant existence form of CH x species. Moreover, CH3OH formation cannot compete with CH x formation on Co( 10 1 ¯ 0 )-A and Co( 10 1 ¯ 1 ) surfaces, considering both surfaces covering 63% of the total surface area exposed of hexahedron Co surfaces, which depends on the reaction conditions, particle size, catalyst support, carbon deposition and many other factors, the contribution to the overall CH x sources from Co( 10 1 ¯ 0 )-A and Co( 10 1 ¯ 1 ) surfaces even surpasses that of other hexahedron Co surfaces under the certain realistic conditions. As a result, the hexahedron Co surfaces exhibit high catalytic selectivity for CH x formation, and provide more CH x sources to participate into the F-T synthesis.
中文翻译:

六面体Co催化剂在费托合成中CHx形成的见解:表面结构对优先机理和存在形式的影响
已经进行了自旋极化密度泛函理论计算,以研究仅在六面体Co(10 1¯0)-A和Co(10 1¯)上的费-托合成中CH x(x = 1–3)形成的优先机理。0)表面。我们的结果表明,与这两个表面上的CO直接解离和氢化成COH相比,CO氢化成CHO更为有利。从C和CHO开始,我们进一步寻找CH x形成的最佳途径,这表明CH x主要是通过Co(10 1¯0)-A表面上的H辅助CO分解途径形成的,其中CH通过CHO离解,CH 2和CH 3通过CH 2形成O分别具有直接解离和H辅助解离;同时,CH 2加氢也有助于CH 3的形成。CH 2和CH 3是Co(10 1¯0)-A表面上的表面富集物质。但是,在Co(10 1¯1)表面上,通过CO直接分解成C,然后连续C氢化,形成CH x物种,C和CH是表面丰富的物种。因此,Co表面结构可以影响CH x物种的优先形成途径和主要存在形式。此外,CH 3 OH的形成不能与CH x竞争考虑到两个表面都覆盖六面体Co表面暴露的总表面积的63%,这取决于Co(10 1¯0)-A和Co(10 1¯1)表面的形成,这取决于反应条件,粒度,催化剂载体由于碳沉积和许多其他因素,在某些实际条件下,Co(10 1¯0)-A和Co(10 1¯1)表面对总CH x源的贡献甚至超过了其他六面体Co表面。结果,六面体Co表面对CH x的形成表现出高的催化选择性,并提供了更多的CH x源以参与FT合成。
更新日期:2016-07-23
中文翻译:

六面体Co催化剂在费托合成中CHx形成的见解:表面结构对优先机理和存在形式的影响
已经进行了自旋极化密度泛函理论计算,以研究仅在六面体Co(10 1¯0)-A和Co(10 1¯)上的费-托合成中CH x(x = 1–3)形成的优先机理。0)表面。我们的结果表明,与这两个表面上的CO直接解离和氢化成COH相比,CO氢化成CHO更为有利。从C和CHO开始,我们进一步寻找CH x形成的最佳途径,这表明CH x主要是通过Co(10 1¯0)-A表面上的H辅助CO分解途径形成的,其中CH通过CHO离解,CH 2和CH 3通过CH 2形成O分别具有直接解离和H辅助解离;同时,CH 2加氢也有助于CH 3的形成。CH 2和CH 3是Co(10 1¯0)-A表面上的表面富集物质。但是,在Co(10 1¯1)表面上,通过CO直接分解成C,然后连续C氢化,形成CH x物种,C和CH是表面丰富的物种。因此,Co表面结构可以影响CH x物种的优先形成途径和主要存在形式。此外,CH 3 OH的形成不能与CH x竞争考虑到两个表面都覆盖六面体Co表面暴露的总表面积的63%,这取决于Co(10 1¯0)-A和Co(10 1¯1)表面的形成,这取决于反应条件,粒度,催化剂载体由于碳沉积和许多其他因素,在某些实际条件下,Co(10 1¯0)-A和Co(10 1¯1)表面对总CH x源的贡献甚至超过了其他六面体Co表面。结果,六面体Co表面对CH x的形成表现出高的催化选择性,并提供了更多的CH x源以参与FT合成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号