当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid–Solid Boundaries Dominate Activity of CO2 Reduction on Gas-Diffusion Electrodes
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-18 , DOI: 10.1021/acscatal.0c03319
Nathan T. Nesbitt 1 , Thomas Burdyny 2 , Hunter Simonson 3, 4 , Danielle Salvatore 3, 4 , Divya Bohra 2 , Recep Kas 1, 4 , Wilson A. Smith 1, 2, 3, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-18 , DOI: 10.1021/acscatal.0c03319
Nathan T. Nesbitt 1 , Thomas Burdyny 2 , Hunter Simonson 3, 4 , Danielle Salvatore 3, 4 , Divya Bohra 2 , Recep Kas 1, 4 , Wilson A. Smith 1, 2, 3, 4
Affiliation
|
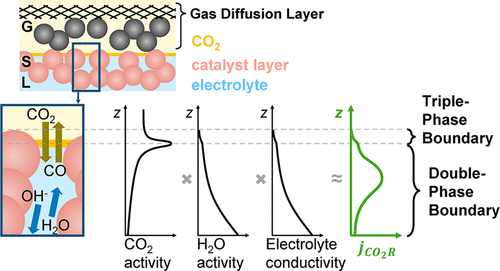
Electrochemical CO2 electrolysis to produce hydrocarbon fuels or material feedstocks offers a renewable alternative to fossilized carbon sources. Gas-diffusion electrodes (GDEs), composed of solid electrocatalysts on porous supports positioned near the interface of a conducting electrolyte and CO2 gas, have been able to demonstrate the substantial current densities needed for future commercialization. These higher reaction rates have often been ascribed to the presence of a three-phase interface, where solid, liquid, and gas provide electrons, water, and CO2, respectively. Conversely, mechanistic work on electrochemical reactions implicates a fully two-phase reaction interface, where gas molecules reach the electrocatalyst’s surface by dissolution and diffusion through the electrolyte. Because the discrepancy between an atomistic three-phase versus two-phase reaction has substantial implications for the design of catalysts, gas-diffusion layers, and cell architectures, the nuances of nomenclatures and governing phenomena surrounding the three-phase-region require clarification. Here we outline the macro, micro, and atomistic phenomena occurring within a gas-diffusion electrode to provide a focused discussion on the architecture of the often-discussed three-phase region for CO2 electrolysis. From this information, we comment on the outlook for the broader CO2 electroreduction GDE cell architecture.
中文翻译:

气固电极上液固边界主导CO 2还原活性
电化学CO 2电解生产碳氢化合物燃料或原料的方法提供了可替代化石碳源的方法。气体扩散电极(GDE)由位于多孔固体载体上的固体电催化剂组成,该多孔催化剂位于导电电解质和CO 2气体的界面附近,已经证明了未来商业化所需的大量电流密度。这些较高的反应速率通常归因于存在三相界面,其中固体,液体和气体会提供电子,水和CO 2, 分别。相反,电化学反应的机理研究涉及完全两相的反应界面,其中气体分子通过在电解质中的溶解和扩散而到达电催化剂的表面。因为原子性三相反应与两相反应之间的差异对催化剂,气体扩散层和电池结构的设计具有实质性影响,所以需要澄清术语的细微差别和围绕三相区域的支配现象。在这里,我们概述了在气体扩散电极中发生的宏观,微观和原子现象,以便对经常讨论的用于CO 2电解的三相区域的体系结构进行重点讨论。从这些信息中,我们对更广泛的CO的前景发表评论2电还原GDE电池架构。
更新日期:2020-12-04
中文翻译:

气固电极上液固边界主导CO 2还原活性
电化学CO 2电解生产碳氢化合物燃料或原料的方法提供了可替代化石碳源的方法。气体扩散电极(GDE)由位于多孔固体载体上的固体电催化剂组成,该多孔催化剂位于导电电解质和CO 2气体的界面附近,已经证明了未来商业化所需的大量电流密度。这些较高的反应速率通常归因于存在三相界面,其中固体,液体和气体会提供电子,水和CO 2, 分别。相反,电化学反应的机理研究涉及完全两相的反应界面,其中气体分子通过在电解质中的溶解和扩散而到达电催化剂的表面。因为原子性三相反应与两相反应之间的差异对催化剂,气体扩散层和电池结构的设计具有实质性影响,所以需要澄清术语的细微差别和围绕三相区域的支配现象。在这里,我们概述了在气体扩散电极中发生的宏观,微观和原子现象,以便对经常讨论的用于CO 2电解的三相区域的体系结构进行重点讨论。从这些信息中,我们对更广泛的CO的前景发表评论2电还原GDE电池架构。

































 京公网安备 11010802027423号
京公网安备 11010802027423号