当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor Microenvironment-Responsive Fe(III)–Porphyrin Nanotheranostics for Tumor Imaging and Targeted Chemodynamic–Photodynamic Therapy
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-11-18 , DOI: 10.1021/acsami.0c14046 Bing Wang 1 , Yeneng Dai 1 , Yingjie Kong 1 , Wenyu Du 1 , Haiyang Ni 1 , Honghai Zhao 1 , Zhiquan Sun 1 , Qingming Shen 1 , Meixing Li 1 , Quli Fan 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-11-18 , DOI: 10.1021/acsami.0c14046 Bing Wang 1 , Yeneng Dai 1 , Yingjie Kong 1 , Wenyu Du 1 , Haiyang Ni 1 , Honghai Zhao 1 , Zhiquan Sun 1 , Qingming Shen 1 , Meixing Li 1 , Quli Fan 1
Affiliation
|
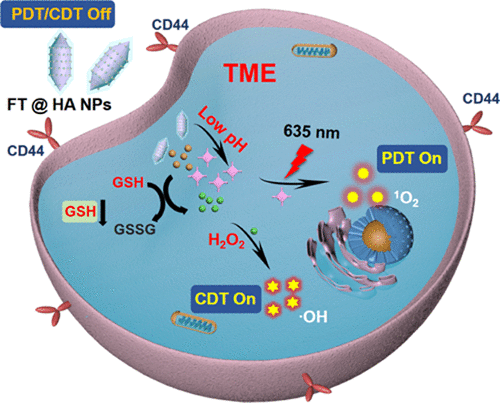
The development of effective and safe tumor nanotheranostics remains a research imperative. Herein, tumor microenvironment (TME)-responsive Fe(III)–porphyrin (TCPP) coordination nanoparticles (FT@HA NPs) were prepared using a simple one-pot method followed by modification with hyaluronic acid (HA). FT@HA NPs specifically accumulated in CD44 receptor-overexpressed tumor tissues through the targeting property of HA and upon endocytosis by tumor cells. After cell internalization, intracellular acidic microenvironments and high levels of glutathione (GSH) triggered the rapid decomposition of FT@HA NPs to release free TCPP molecules and Fe(III) ions. The released Fe(III) ions could trigger GSH depletion and Fenton reaction, activating chemodynamic therapy (CDT). Meanwhile, the fluorescence and photodynamic effects of the TCPP could be also activated, achieving controlled reactive oxygen species (ROS) generation and avoiding side effects on normal tissues. Moreover, the rapid consumption of GSH further enhanced the efficacy of CDT and photodynamic therapy (PDT). The in vivo experiments further demonstrated that the antitumor effect of these nanotheranostics was significantly enhanced and that their toxicity and side effects against normal tissues were effectively suppressed. The FT@HA NPs can be applied for activated tumor combination therapy under the guidance of dual-mode imaging including fluorescence imaging and magnetic resonance imaging, providing an effective strategy for the design and preparation of TME-responsive multifunctional nanotheranostics for precise tumor imaging and combination therapy.
中文翻译:

用于肿瘤成像和靶向化学动力学-光动力疗法的肿瘤微环境响应性 Fe(III)-卟啉纳米诊断学
开发有效且安全的肿瘤纳米治疗药物仍然是一项研究当务之急。在此,使用简单的一锅法制备肿瘤微环境(TME)响应性铁(III)-卟啉(TCPP)配位纳米颗粒(FT@HA NPs),然后用透明质酸(HA)修饰。FT@HA NPs 通过 HA 的靶向特性和肿瘤细胞的内吞作用特异性地积累在 CD44 受体过表达的肿瘤组织中。细胞内化后,细胞内酸性微环境和高水平的谷胱甘肽 (GSH) 引发 FT@HA NPs 的快速分解,释放出游离的 TCPP 分子和 Fe(III) 离子。释放的 Fe(III) 离子可触发 GSH 耗竭和 Fenton 反应,从而激活化学动力学疗法 (CDT)。同时,TCPP的荧光和光动力效应也可以被激活,实现受控的活性氧 (ROS) 生成并避免对正常组织的副作用。此外,GSH 的快速消耗进一步增强了 CDT 和光动力疗法 (PDT) 的疗效。这体内实验进一步证明,这些纳米治疗药物的抗肿瘤作用显着增强,并且对正常组织的毒副作用得到有效抑制。FT@HA NPs可在荧光成像和磁共振成像等双模成像的指导下应用于活化肿瘤联合治疗,为设计和制备TME响应的多功能纳米治疗药物提供了一种有效的策略,用于肿瘤的精确成像和联合治疗治疗。
更新日期:2020-11-18
中文翻译:

用于肿瘤成像和靶向化学动力学-光动力疗法的肿瘤微环境响应性 Fe(III)-卟啉纳米诊断学
开发有效且安全的肿瘤纳米治疗药物仍然是一项研究当务之急。在此,使用简单的一锅法制备肿瘤微环境(TME)响应性铁(III)-卟啉(TCPP)配位纳米颗粒(FT@HA NPs),然后用透明质酸(HA)修饰。FT@HA NPs 通过 HA 的靶向特性和肿瘤细胞的内吞作用特异性地积累在 CD44 受体过表达的肿瘤组织中。细胞内化后,细胞内酸性微环境和高水平的谷胱甘肽 (GSH) 引发 FT@HA NPs 的快速分解,释放出游离的 TCPP 分子和 Fe(III) 离子。释放的 Fe(III) 离子可触发 GSH 耗竭和 Fenton 反应,从而激活化学动力学疗法 (CDT)。同时,TCPP的荧光和光动力效应也可以被激活,实现受控的活性氧 (ROS) 生成并避免对正常组织的副作用。此外,GSH 的快速消耗进一步增强了 CDT 和光动力疗法 (PDT) 的疗效。这体内实验进一步证明,这些纳米治疗药物的抗肿瘤作用显着增强,并且对正常组织的毒副作用得到有效抑制。FT@HA NPs可在荧光成像和磁共振成像等双模成像的指导下应用于活化肿瘤联合治疗,为设计和制备TME响应的多功能纳米治疗药物提供了一种有效的策略,用于肿瘤的精确成像和联合治疗治疗。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号