当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reshaping the Binding Pocket of Lysine Hydroxylase for Enhanced Activity
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-16 , DOI: 10.1021/acscatal.0c03841 Fenghua Wang 1 , Menglu Zhu 1 , Zhan Song 1 , Chao Li 1 , Yuying Wang 1 , Zhangliang Zhu 1 , Dengyue Sun 2 , Fuping Lu 1 , Hui-Min Qin 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-16 , DOI: 10.1021/acscatal.0c03841 Fenghua Wang 1 , Menglu Zhu 1 , Zhan Song 1 , Chao Li 1 , Yuying Wang 1 , Zhangliang Zhu 1 , Dengyue Sun 2 , Fuping Lu 1 , Hui-Min Qin 1
Affiliation
|
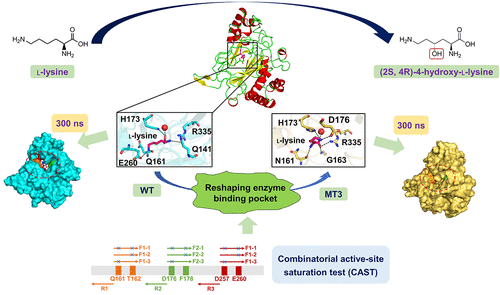
The versatile synthetic intermediate (2S,4R)-4-hydroxylysine can be produced using l-lysine hydroxylase. However, the wild-type enzyme cannot effectively catalyze the C4 hydroxylation of l-lysine to form the product. To overcome this bottleneck, we modified the l-lysine hydroxylase from Niastella koreensis (NkLH4), using the semirational combinatorial active-site saturation test (CAST). We obtained a highly active mutant MT3 (Q161N/T162A/F178Y/E260D) with a 24.97-fold increase of kcat/Km, compared with the wild-type enzyme (791.33 mM–1 s–1 vs 31.69 mM–1 s–1). Further analysis of the structure–activity relationship via molecular dynamics (MD) simulations suggested that MT3 had a more flexible conformation, as well as an enlarged substrate-binding pocket with decreased steric hindrance and increased binding energy in substrate recognition. Our study provides a highly active NkLH4 mutant for potential commercial use in the production of enantiomerically pure (2S,4R)-4-hydroxylysine.
中文翻译:

重塑赖氨酸羟化酶的结合口袋以增强活性
通用的合成中间体(2 S,4 R)-4-羟基赖氨酸可以使用l-赖氨酸羟化酶生产。然而,野生型酶不能有效催化1-赖氨酸的C4羟基化以形成产物。为了克服这个瓶颈,我们使用半定量组合活性位饱和度测试(CAST)修改了来自日本猪笼草(Nastella koreensis)的l-赖氨酸羟化酶(NkLH4)。我们获得了一个高活性突变体MT3(Q161N / T162A / F178Y / E260D),与野生型酶(791.33 mM –1 s –1 vs 31.69 mM)相比,k cat / K m增加了24.97倍。–1 s –1)。通过分子动力学(MD)模拟对结构-活性关系的进一步分析表明,MT3具有更灵活的构象,以及扩大的底物结合口袋,空间位阻降低,结合能在底物识别中增加。我们的研究提供了一种高活性NkLH4突变体,可用于生产对映体纯的(2 S,4 R)-4-羟基赖氨酸。
更新日期:2020-12-04
中文翻译:

重塑赖氨酸羟化酶的结合口袋以增强活性
通用的合成中间体(2 S,4 R)-4-羟基赖氨酸可以使用l-赖氨酸羟化酶生产。然而,野生型酶不能有效催化1-赖氨酸的C4羟基化以形成产物。为了克服这个瓶颈,我们使用半定量组合活性位饱和度测试(CAST)修改了来自日本猪笼草(Nastella koreensis)的l-赖氨酸羟化酶(NkLH4)。我们获得了一个高活性突变体MT3(Q161N / T162A / F178Y / E260D),与野生型酶(791.33 mM –1 s –1 vs 31.69 mM)相比,k cat / K m增加了24.97倍。–1 s –1)。通过分子动力学(MD)模拟对结构-活性关系的进一步分析表明,MT3具有更灵活的构象,以及扩大的底物结合口袋,空间位阻降低,结合能在底物识别中增加。我们的研究提供了一种高活性NkLH4突变体,可用于生产对映体纯的(2 S,4 R)-4-羟基赖氨酸。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号