当前位置:
X-MOL 学术
›
Batteries Supercaps
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Singlet Oxygen in Lithium−Oxygen Batteries
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-11-17 , DOI: 10.1002/batt.202000210 Misun Hong 1 , Hye Ryung Byon 2, 3
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-11-17 , DOI: 10.1002/batt.202000210 Misun Hong 1 , Hye Ryung Byon 2, 3
Affiliation
|
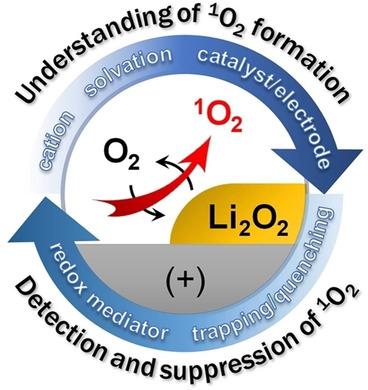
Singlet oxygen (1O2) is one of the most critical species leading to parasitic side reactions and poor reversibility in non‐aqueous Li−O2 batteries. 1O2 is generated via the disproportionation of the superoxide radical (O2.−) in O2/Li2O2 electrochemistry. The mechanistic and computational studies on 1O2 formation revealed the significant roles of the associated cations, solvation ability of aprotic solvents, H+ source, and catalyst/electrode materials. Along with efforts to alleviate 1O2 production, trapping and eliminating 1O2 have been attempted using molecular agents. Anthracene derivatives trap 1O2 and form endoperoxides, which can be quantitatively detected using in situ fluorescence analysis. Physical quenchers that convert 1O2 to 3O2 are desirable for cycling of Li−O2 cells because quencher molecules are reusable. We highlight the recent reports on the formation and elimination of 1O2, and challenges and perspectives of suppressing the 1O2 effect on the performance of Li−O2 cells.
中文翻译:

锂氧电池中的单重态氧
单线态氧(1 O 2)是导致非水Li-O 2电池中寄生副反应和不良可逆性的最关键物质之一。通过O 2 / Li 2 O 2电化学中超氧自由基(O 2 .-)的歧化产生1 O 2。对1 O 2形成的机理和计算研究表明,相关阳离子的重要作用,非质子溶剂的溶剂化能力,H +源和催化剂/电极材料。努力减轻1 O已经尝试使用分子试剂来产生2种,捕获和消除1 O 2。蒽衍生物捕获1 O 2并形成内过氧化物,可使用原位荧光分析法定量检测。转换1 O 2到3 O 2的物理淬灭剂对于Li-O 2电池的循环是理想的,因为淬灭剂分子是可重复使用的。我们重点介绍了有关1 O 2形成和消除的最新报告,以及抑制1 O 2的挑战和观点。对Li-O 2电池性能的影响。
更新日期:2020-11-17
中文翻译:

锂氧电池中的单重态氧
单线态氧(1 O 2)是导致非水Li-O 2电池中寄生副反应和不良可逆性的最关键物质之一。通过O 2 / Li 2 O 2电化学中超氧自由基(O 2 .-)的歧化产生1 O 2。对1 O 2形成的机理和计算研究表明,相关阳离子的重要作用,非质子溶剂的溶剂化能力,H +源和催化剂/电极材料。努力减轻1 O已经尝试使用分子试剂来产生2种,捕获和消除1 O 2。蒽衍生物捕获1 O 2并形成内过氧化物,可使用原位荧光分析法定量检测。转换1 O 2到3 O 2的物理淬灭剂对于Li-O 2电池的循环是理想的,因为淬灭剂分子是可重复使用的。我们重点介绍了有关1 O 2形成和消除的最新报告,以及抑制1 O 2的挑战和观点。对Li-O 2电池性能的影响。






























 京公网安备 11010802027423号
京公网安备 11010802027423号