当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Local structure of a highly concentrated NaClO4 aqueous solution-type electrolyte for sodium ion batteries
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-10-29 , DOI: 10.1039/d0cp04376a
Ryo Sakamoto 1, 2, 3, 4 , Maho Yamashita 4, 5, 6, 7 , Kosuke Nakamoto 2, 3, 4, 8 , Yongquan Zhou 9, 10, 11, 12 , Nobuko Yoshimoto 4, 5, 6, 7 , Kenta Fujii 4, 5, 6, 7 , Toshio Yamaguchi 4, 13, 14, 15, 16 , Ayuko Kitajou 4, 5, 6, 7, 17 , Shigeto Okada 2, 3, 4, 8, 17
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-10-29 , DOI: 10.1039/d0cp04376a
Ryo Sakamoto 1, 2, 3, 4 , Maho Yamashita 4, 5, 6, 7 , Kosuke Nakamoto 2, 3, 4, 8 , Yongquan Zhou 9, 10, 11, 12 , Nobuko Yoshimoto 4, 5, 6, 7 , Kenta Fujii 4, 5, 6, 7 , Toshio Yamaguchi 4, 13, 14, 15, 16 , Ayuko Kitajou 4, 5, 6, 7, 17 , Shigeto Okada 2, 3, 4, 8, 17
Affiliation
|
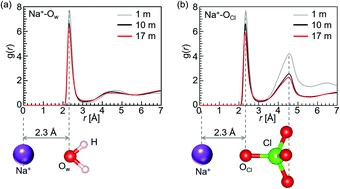
Aqueous Na-ion batteries with highly concentrated NaClO4 aq. electrolytes are drawing attention as candidates for large-scale rechargeable batteries with a high safety level. However, the detailed mechanism by which the potential window in 17 m NaClO4 aq. electrolyte was expanded remains unclear. Therefore, we investigated the local structure around a Na+ ion or a ClO4− ion using X-ray diffraction combined with empirical potential structure refinement (EPSR) modelling and Raman spectroscopy. The results showed that in 17 m NaClO4 aq. electrolyte, most of the water molecules were coordinated to Na+ ions and few free water molecules were present. The 17 m NaClO4 aq. electrolyte could be interpreted as widening the potential window because almost all water molecules participated in hydration of the Na+ ions.
中文翻译:

用于钠离子电池的高浓度NaClO4水溶液型电解质的局部结构
含高浓度NaClO 4水溶液的Na离子水溶液。电解质作为具有高安全等级的大型可再充电电池的候选者而受到关注。但是,详细的机理是通过在17 m NaClO 4水溶液中的电位窗口实现的。电解质是否膨胀仍不清楚。因此,我们研究周围中的Na的局部结构+离子或CLO 4 -建模和拉曼光谱离子使用X射线衍射用经验势结构精修(EPSR)相结合。结果表明,在17 m NaClO 4水溶液中。电解质中,大多数水分子与Na +离子配位,并且几乎没有游离水分子存在。17 m NaClO4平方米 电解质可以解释为扩大了电位窗口,因为几乎所有水分子都参与了Na +离子的水合作用。
更新日期:2020-11-13
中文翻译:

用于钠离子电池的高浓度NaClO4水溶液型电解质的局部结构
含高浓度NaClO 4水溶液的Na离子水溶液。电解质作为具有高安全等级的大型可再充电电池的候选者而受到关注。但是,详细的机理是通过在17 m NaClO 4水溶液中的电位窗口实现的。电解质是否膨胀仍不清楚。因此,我们研究周围中的Na的局部结构+离子或CLO 4 -建模和拉曼光谱离子使用X射线衍射用经验势结构精修(EPSR)相结合。结果表明,在17 m NaClO 4水溶液中。电解质中,大多数水分子与Na +离子配位,并且几乎没有游离水分子存在。17 m NaClO4平方米 电解质可以解释为扩大了电位窗口,因为几乎所有水分子都参与了Na +离子的水合作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号