当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of (S)-2-(1-(4-Amino-3-(3-fluoro-4-methoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)propyl)-3-cyclopropyl-5-fluoroquinazolin-4(3H)-one (IHMT-PI3Kδ-372) as a Potent and Selective PI3Kδ Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jmedchem.0c01544 Feng Li 1, 2 , Xiaofei Liang 1, 3 , Zongru Jiang 1, 3 , Aoli Wang 1, 3 , Junjie Wang 1, 2 , Cheng Chen 1, 2 , Wenliang Wang 1, 2 , Fengming Zou 1, 3 , Ziping Qi 1, 3 , Qingwang Liu 1, 3 , Zhenquan Hu 1 , Jiangyan Cao 1, 2 , Hong Wu 1, 3 , Beilei Wang 1, 3 , Li Wang 1, 3 , Jing Liu 1, 3, 4 , Qingsong Liu 1, 2, 3, 4, 5
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jmedchem.0c01544 Feng Li 1, 2 , Xiaofei Liang 1, 3 , Zongru Jiang 1, 3 , Aoli Wang 1, 3 , Junjie Wang 1, 2 , Cheng Chen 1, 2 , Wenliang Wang 1, 2 , Fengming Zou 1, 3 , Ziping Qi 1, 3 , Qingwang Liu 1, 3 , Zhenquan Hu 1 , Jiangyan Cao 1, 2 , Hong Wu 1, 3 , Beilei Wang 1, 3 , Li Wang 1, 3 , Jing Liu 1, 3, 4 , Qingsong Liu 1, 2, 3, 4, 5
Affiliation
|
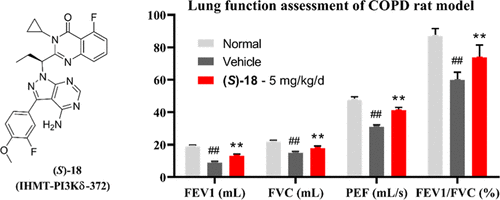
Accumulated pieces of evidence have shown that PI3Kδ plays a critical role in chronic obstructive pulmonary disease (COPD). Using a fragment-hybrid approach, we discovered a potent and selective PI3Kδ inhibitor (S)-18. In the biochemical assay, (S)-18 inhibits PI3Kδ (IC50 = 14 nM) with high selectivity over other class I PI3Ks (56∼83 fold). (S)-18 also achieves good selectivity over other protein kinases in the kinome (S-score (35) = 0.015). In the cell, (S)-18 selectively and potently inhibits the PI3Kδ-mediated phosphorylation of AKT T308 but not other class I PI3K-mediated signaling. Additionally, (S)-18 exhibits no apparent inhibitory effect on CYP isoforms except for a moderate effect on CYP2C9. Furthermore, it shows no apparent inhibitory activity against hERG (IC50 > 10 μM). In vivo, (S)-18 displays favorable PK properties for inhaled delivery and improves lung function in a rodent model of pulmonary inflammation. These results suggest that (S)-18 might be a new potential therapeutic candidate for COPD.
中文翻译:

(S)-2-(1-(4-氨基-3-(3-氟-4-甲氧基苯基)-1 H-吡唑并[3,4- d ]嘧啶-1-基)丙基)-3-的发现环丙基-5-氟喹唑啉-4(3 H)-1(IHMT-PI3Kδ-372)作为有效的选择性PI3Kδ抑制剂,用于治疗慢性阻塞性肺疾病
越来越多的证据表明,PI3Kδ在慢性阻塞性肺疾病(COPD)中起关键作用。使用片段杂交方法,我们发现了有效的选择性PI3Kδ抑制剂(S)-18。在生化分析中,(S)-18抑制PI3Kδ(IC 50 = 14 nM)的选择性比其他I类PI3K高(56〜83倍)。(S)-18也比激酶组中的其他蛋白激酶具有更好的选择性(S得分(35)= 0.015)。在单元格中,(S)-18选择性和有效地抑制PI3Kδ介导的AKT T308磷酸化,但不抑制其他I类PI3K介导的信号转导。此外,(S)-18除对CYP2C9有中等作用外,对CYP亚型没有明显的抑制作用。此外,它没有显示出对hERG的明显抑制活性(IC 50 > 10μM)。在体内,(S)-18在吸入性啮齿动物肺炎模型中显示出良好的PK吸入吸入特性,并改善了肺功能。这些结果表明,(S)-18可能是COPD的一种新的潜在治疗候选物。
更新日期:2020-11-25
中文翻译:

(S)-2-(1-(4-氨基-3-(3-氟-4-甲氧基苯基)-1 H-吡唑并[3,4- d ]嘧啶-1-基)丙基)-3-的发现环丙基-5-氟喹唑啉-4(3 H)-1(IHMT-PI3Kδ-372)作为有效的选择性PI3Kδ抑制剂,用于治疗慢性阻塞性肺疾病
越来越多的证据表明,PI3Kδ在慢性阻塞性肺疾病(COPD)中起关键作用。使用片段杂交方法,我们发现了有效的选择性PI3Kδ抑制剂(S)-18。在生化分析中,(S)-18抑制PI3Kδ(IC 50 = 14 nM)的选择性比其他I类PI3K高(56〜83倍)。(S)-18也比激酶组中的其他蛋白激酶具有更好的选择性(S得分(35)= 0.015)。在单元格中,(S)-18选择性和有效地抑制PI3Kδ介导的AKT T308磷酸化,但不抑制其他I类PI3K介导的信号转导。此外,(S)-18除对CYP2C9有中等作用外,对CYP亚型没有明显的抑制作用。此外,它没有显示出对hERG的明显抑制活性(IC 50 > 10μM)。在体内,(S)-18在吸入性啮齿动物肺炎模型中显示出良好的PK吸入吸入特性,并改善了肺功能。这些结果表明,(S)-18可能是COPD的一种新的潜在治疗候选物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号