当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of Hydrogen Peroxide by a Titanium Oxide-Supported Iron Catalyst: Evidence for Surface Fe(IV) and Its Selectivity
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.est.0c04262 Hak-Hyeon Kim 1 , Hongshin Lee 2 , Donghyun Lee 3 , Young-Jin Ko 4 , Heesoo Woo 5 , Jaesang Lee 2 , Changha Lee 3 , Anh Le-Tuan Pham 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.est.0c04262 Hak-Hyeon Kim 1 , Hongshin Lee 2 , Donghyun Lee 3 , Young-Jin Ko 4 , Heesoo Woo 5 , Jaesang Lee 2 , Changha Lee 3 , Anh Le-Tuan Pham 1
Affiliation
|
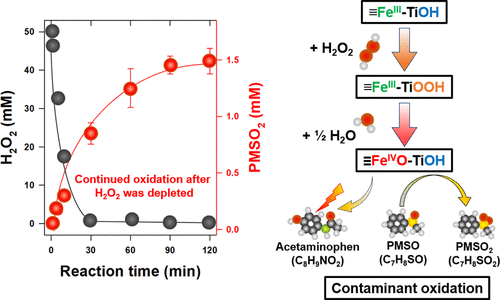
Iron immobilized on supports such as silica, alumina, titanium oxide, and zeolite can activate hydrogen peroxide (H2O2) into strong oxidants. However, the role of the support and the nature of the oxidants produced in this process remain elusive. This study investigated the activation of H2O2 by a TiO2-supported catalyst (FeTi-ox). Characterizing the catalyst surface in situ using X-ray absorption spectroscopy (XAS), together with X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR), revealed that the interaction between H2O2 and the TiO2 phase played a key role in the H2O2 activation. This interaction generated a stable peroxo–titania ≡Fe(III)–Ti–OOH complex, which reacted further with H2O to produce a surface oxidant, likely ≡Fe[IV] ═ O2+. The oxidant effectively degraded acetaminophen, even in the presence of chloride, bicarbonate, and organic matter. Unexpectedly, contaminant oxidation continued after the H2O2 in the solution was depleted, owing to the decomposition of ≡Fe(III)–Ti–OOH by water. In addition, the FeTi-ox catalyst effectively degraded acetaminophen over five testing cycles. Overall, new insights gained in this study may provide a basis for designing more effective catalysts for H2O2 activation.
中文翻译:

氧化钛负载的铁催化剂对过氧化氢的活化作用:表面Fe(IV)及其选择性的证据
固定在二氧化硅,氧化铝,氧化钛和沸石等载体上的铁可将过氧化氢(H 2 O 2)活化为强氧化剂。然而,在该过程中载体的作用和氧化剂的性质仍然难以捉摸。本研究研究了TiO 2负载的催化剂(FeTi-ox)对H 2 O 2的活化作用。使用X射线吸收光谱(XAS),X射线光电子能谱(XPS)和电子顺磁共振(EPR)来原位表征催化剂表面,表明H 2 O 2和TiO 2相之间的相互作用起到了H 2 O中的关键作用2激活。这种相互作用产生了稳定的过氧-二氧化钛≡Fe(III)-Ti-OOH配合物,它与H 2 O进一步反应生成表面氧化剂,可能是≡Fe[IV]═O2 +。即使在存在氯化物,碳酸氢盐和有机物的情况下,氧化剂也能有效降解对乙酰氨基酚。出乎意料的是,由于水分解≡Fe(III)-Ti-OOH,溶液中的H 2 O 2耗尽后,污染物继续氧化。此外,FeTi-ox催化剂可在五个测试周期内有效降解对乙酰氨基酚。总体而言,在这项研究中获得的新见解可能为设计更有效的H 2 O 2活化催化剂提供基础。
更新日期:2020-12-01
中文翻译:

氧化钛负载的铁催化剂对过氧化氢的活化作用:表面Fe(IV)及其选择性的证据
固定在二氧化硅,氧化铝,氧化钛和沸石等载体上的铁可将过氧化氢(H 2 O 2)活化为强氧化剂。然而,在该过程中载体的作用和氧化剂的性质仍然难以捉摸。本研究研究了TiO 2负载的催化剂(FeTi-ox)对H 2 O 2的活化作用。使用X射线吸收光谱(XAS),X射线光电子能谱(XPS)和电子顺磁共振(EPR)来原位表征催化剂表面,表明H 2 O 2和TiO 2相之间的相互作用起到了H 2 O中的关键作用2激活。这种相互作用产生了稳定的过氧-二氧化钛≡Fe(III)-Ti-OOH配合物,它与H 2 O进一步反应生成表面氧化剂,可能是≡Fe[IV]═O2 +。即使在存在氯化物,碳酸氢盐和有机物的情况下,氧化剂也能有效降解对乙酰氨基酚。出乎意料的是,由于水分解≡Fe(III)-Ti-OOH,溶液中的H 2 O 2耗尽后,污染物继续氧化。此外,FeTi-ox催化剂可在五个测试周期内有效降解对乙酰氨基酚。总体而言,在这项研究中获得的新见解可能为设计更有效的H 2 O 2活化催化剂提供基础。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号