当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Immolative Difluorophenyl Ester Linker for Affinity-Based Fluorescence Turn-on Protein Detection
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.analchem.0c03178 Hsiang-Yun Huang, Syuan-Yun Fan, En-Hao Chang, Chak Hin Lam, Yu-Chun Lin, Xin-Hui Lin, Nitesh K. Gupta, Kui-Thong Tan
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.analchem.0c03178 Hsiang-Yun Huang, Syuan-Yun Fan, En-Hao Chang, Chak Hin Lam, Yu-Chun Lin, Xin-Hui Lin, Nitesh K. Gupta, Kui-Thong Tan
|
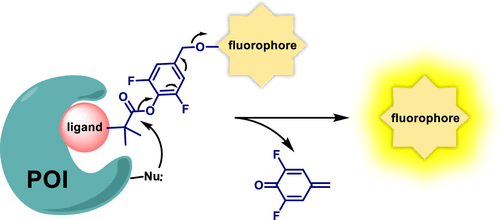
Currently most fluorogenic probes are developed for the analysis of enzymes, where a bond breaking or rearrangement reaction is required to transform a nonfluorescent enzymatic substrate into a fluorescent product. However, this approach cannot be used for proteins that do not possess enzymatic activities. In this article, we show that fluorogenic probes with a self-immolative difluorophenyl ester linker can mimic the bond disassembly processes of fluorogenic enzyme substrates for the rapid analysis of nonenzymatic proteins. Although numerous self-immolative reagents have shown promising applications in sensors, drug delivery systems, and material chemistry, all of them are triggered by either enzymes or small reactive molecules. In our strategy, the probe binds to the protein via a specific protein–ligand interaction, inducing a chemical reaction between the self-immolative linker and an amino acid of the protein, thereby triggering a cascade reaction that leads to the activation and release of the fluorogenic reporter. In contrast, a phenyl ester linker without the difluoro substituent cannot be triggered to release the fluorogenic reporter. With this probe design, live-cell imaging of extracellular and intracellular endogenous tumor marker proteins can be achieved with high selectivity and sensitivity.
中文翻译:

用于基于亲和力的荧光开启蛋白检测的自消灭二氟苯基酯接头
当前,大多数荧光探针被开发用于酶的分析,其中需要键断裂或重排反应以将非荧光酶促底物转化为荧光产物。但是,这种方法不能用于不具有酶促活性的蛋白质。在本文中,我们显示了具有自消灭性二氟苯基酯接头的荧光探针可以模拟荧光酶底物的键分解过程,以快速分析非酶蛋白。尽管许多自消灭试剂已在传感器,药物输送系统和材料化学中显示出广阔的应用前景,但它们全都被酶或小的反应分子触发。在我们的策略中,探针通过特定的蛋白质-配体相互作用与蛋白质结合,诱导自消灭性接头与蛋白质氨基酸之间的化学反应,从而触发级联反应,从而导致荧光报告基因的活化和释放。相反,没有二氟取代基的苯酯连接基不能被触发释放荧光报告基团。通过这种探针设计,可以高选择性和高灵敏度实现细胞外和细胞内内源性肿瘤标志物蛋白的活细胞成像。
更新日期:2020-12-01
中文翻译:

用于基于亲和力的荧光开启蛋白检测的自消灭二氟苯基酯接头
当前,大多数荧光探针被开发用于酶的分析,其中需要键断裂或重排反应以将非荧光酶促底物转化为荧光产物。但是,这种方法不能用于不具有酶促活性的蛋白质。在本文中,我们显示了具有自消灭性二氟苯基酯接头的荧光探针可以模拟荧光酶底物的键分解过程,以快速分析非酶蛋白。尽管许多自消灭试剂已在传感器,药物输送系统和材料化学中显示出广阔的应用前景,但它们全都被酶或小的反应分子触发。在我们的策略中,探针通过特定的蛋白质-配体相互作用与蛋白质结合,诱导自消灭性接头与蛋白质氨基酸之间的化学反应,从而触发级联反应,从而导致荧光报告基因的活化和释放。相反,没有二氟取代基的苯酯连接基不能被触发释放荧光报告基团。通过这种探针设计,可以高选择性和高灵敏度实现细胞外和细胞内内源性肿瘤标志物蛋白的活细胞成像。































 京公网安备 11010802027423号
京公网安备 11010802027423号