Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-11-11 , DOI: 10.1016/j.jconrel.2020.11.016
Wenmin Yuan 1 , Bilian Yu 2 , Minzhi Yu 1 , Rui Kuai 3 , Emily E Morin 1 , Huilun Wang 4 , Die Hu 4 , Jifeng Zhang 4 , James J Moon 5 , Y Eugene Chen 4 , Yanhong Guo 4 , Anna Schwendeman 6
|
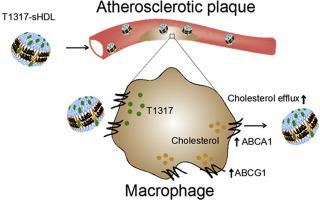
Liver X nuclear receptor (LXR) agonists are promising anti-atherosclerotic agents that increase the expression of cholesterol transporters on atheroma macrophages leading to increased efflux of cholesterol to endogenous high-density lipoprotein (HDL) acceptors. HDL subsequently delivers effluxed cholesterol to the liver by the process of reverse cholesterol transport, resulting in reduction of atherosclerotic plaques. However, LXR agonists administration triggers undesirable liver steatosis and hypertriglyceridemia due to increased fatty acid and sterol synthesis. LXR-induced liver toxicity, poor drug aqueous solubility and low levels of endogenous HDL acceptors in target patient populations limit the clinical translation of LXR agonists. Here, we propose a dual-antiatherogenic strategy for administration of the LXR agonist, T0901317 (T1317), by encapsulating in synthetic HDL (sHDL) nanoparticles. sHDL had been clinically proven to serve as cholesterol acceptors, resulting in plaque reduction in atherosclerosis patients. In addition, the hydrophobic core and endogenous atheroma-targeting ability of sHDL allow for encapsulation of water-insoluble drugs and their subsequent delivery to atheroma. Several compositions of sHDL were tested to optimize both T1317 encapsulation efficiency and ability of T1317-sHDL to efflux cholesterol. Optimized T1317-sHDL exhibited more efficient cholesterol efflux from macrophages and enhanced atheroma-targeting relative to free drug. Most importantly, in an apolipoprotein E deficient (ApoE−/−) atherosclerosis progression murine model, T1317-sHDL showed superior inhibition of atherogenesis and reduced hypertriglyceridemia side effects in comparison to the free drug and blank sHDL. The T1317-sHDL pharmacological efficacy was observed at doses lower than those previously described for LXR agents, which may have additional safety benefits. In addition, the established clinical manufacturing, safety and efficacy of blank sHDL nanoparticles used in this study could facilitate future clinical translation of LXR-loaded sHDLs.
中文翻译:

合成高密度脂蛋白传递肝脏 X 受体激动剂,通过增强反向胆固醇转运来预防动脉粥样硬化形成
肝脏 X 核受体 (LXR) 激动剂是有前途的抗动脉粥样硬化药物,可增加动脉粥样硬化巨噬细胞上胆固醇转运蛋白的表达,从而增加胆固醇向内源性高密度脂蛋白 (HDL) 受体的流出。HDL 随后通过反向胆固醇转运过程将流出的胆固醇输送至肝脏,从而减少动脉粥样硬化斑块。然而,由于脂肪酸和甾醇合成增加,LXR激动剂给药会引发不良的肝脏脂肪变性和高甘油三酯血症。LXR 诱导的肝毒性、药物水溶性差以及目标患者群体中内源性 HDL 受体水平低限制了 LXR 激动剂的临床转化。在这里,我们提出了一种双重抗动脉粥样硬化策略,通过封装在合成 HDL (sHDL) 纳米颗粒中来施用 LXR 激动剂 T0901317 (T1317)。临床证明 sHDL 可以作为胆固醇受体,导致动脉粥样硬化患者的斑块减少。此外,sHDL 的疏水核心和内源性动脉粥样硬化斑块靶向能力允许封装水不溶性药物并随后将其递送至动脉粥样硬化斑块。测试了几种 sHDL 组合物,以优化 T1317 封装效率和 T1317-sHDL 流出胆固醇的能力。相对于游离药物,优化的 T1317-sHDL 表现出更有效的巨噬细胞胆固醇流出和增强的动脉粥样硬化靶向性。最重要的是,在载脂蛋白 E 缺陷 (ApoE −/− ) 动脉粥样硬化进展小鼠模型中,与游离药物和空白 sHDL 相比,T1317-sHDL 显示出优异的动脉粥样硬化抑制作用并降低了高甘油三酯血症副作用。在低于之前描述的 LXR 药物的剂量下观察到 T1317-sHDL 药理功效,这可能具有额外的安全益处。此外,本研究中使用的空白 sHDL 纳米颗粒已建立的临床制造、安全性和功效可以促进 LXR 负载 sHDL 的未来临床转化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号