当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unified Strategy to Amphenicol Antibiotics: Asymmetric Synthesis of (−)-Chloramphenicol, (−)-Azidamphenicol, and (+)-Thiamphenicol and Its (+)-3-Floride
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.joc.0c02181 Jinxin Liu 1 , Yaling Li 1 , Miaolin Ke 2 , Minjie Liu 2 , Pingping Zhan 2 , You-Cai Xiao 1 , Fener Chen 1, 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.joc.0c02181 Jinxin Liu 1 , Yaling Li 1 , Miaolin Ke 2 , Minjie Liu 2 , Pingping Zhan 2 , You-Cai Xiao 1 , Fener Chen 1, 2, 3
Affiliation
|
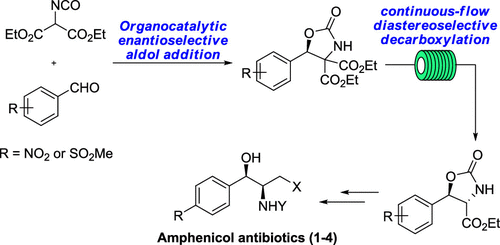
The asymmetric synthesis of (−)-chloramphenicol, (−)-azidamphenicol, and (+)-thiamphenicol and its (+)-3-floride, (+)-florfenicol, is reported. This approach toward the amphenicol antibiotic family features two key steps: (1) a cinchona alkaloid derived urea-catalyzed aldol reaction allows highly enantioselective access to oxazolidinone gem-diesters and (2) a continuous flow diastereoselective decarboxylation of thermally stable oxazolidinone gem-diesters to form the desired trans-oxazolidinone monoesters with two adjacent stereocenters that provide the desired privileged scaffolds of syn-vicinal amino alcohols in the amphenicol family.
中文翻译:

氨苯酚类抗生素的统一策略:(-)-氯霉素,(-)-阿达木酚和(+)-甲砜霉素及其(+)-3-氟化物的不对称合成
报道了(-)-氯霉素,(-)-阿达木酚和(+)-噻吩酚及其(+)-3-小肽,(+)-氟苯尼考的不对称合成。这种对苯甲酚类抗生素家族的方法具有两个关键步骤:(1)金鸡纳生物碱衍生的尿素催化的醛醇缩合反应可实现对映体对恶唑烷酮宝石二酯的高度对映选择性;(2)将热稳定的恶唑烷酮宝石二酯连续流非对映选择性脱羧为形成所需的反式与提供的所需特权支架两个相邻的立体-oxazolidinone单酯顺-vicinal氨基醇在amphenicol家族。
更新日期:2020-12-04
中文翻译:

氨苯酚类抗生素的统一策略:(-)-氯霉素,(-)-阿达木酚和(+)-甲砜霉素及其(+)-3-氟化物的不对称合成
报道了(-)-氯霉素,(-)-阿达木酚和(+)-噻吩酚及其(+)-3-小肽,(+)-氟苯尼考的不对称合成。这种对苯甲酚类抗生素家族的方法具有两个关键步骤:(1)金鸡纳生物碱衍生的尿素催化的醛醇缩合反应可实现对映体对恶唑烷酮宝石二酯的高度对映选择性;(2)将热稳定的恶唑烷酮宝石二酯连续流非对映选择性脱羧为形成所需的反式与提供的所需特权支架两个相邻的立体-oxazolidinone单酯顺-vicinal氨基醇在amphenicol家族。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号