Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation Mechanism of Ammonium Carbamate for CO2 Uptake in N,N′-Dimethylethylenediamine Grafted M2(dobpdc)
Langmuir ( IF 3.7 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.langmuir.0c02750 Hui Zhang 1 , Li-Ming Yang 1 , Eric Ganz 2
Langmuir ( IF 3.7 ) Pub Date : 2020-11-10 , DOI: 10.1021/acs.langmuir.0c02750 Hui Zhang 1 , Li-Ming Yang 1 , Eric Ganz 2
Affiliation
|
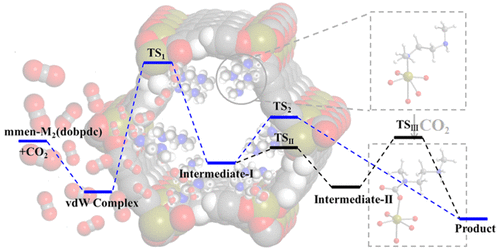
The adsorption properties and formation mechanism of ammonium carbamate for CO2 capture in N,N′-dimethylethylenediamine (mmen) grafted M2(dobpdc) (dobpdc4– = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate; M = Mg, Sc–Zn, except Ni) have been studied via density functional theory (DFT) calculations. We see that the mmen molecule is joined to the metal site via a M–N bond and has hydrogen bonding with neighboring mmen molecules. The binding energies of mmen range from 135.4 to 184.0 kJ/mol. CO2 is captured via insertion into the M–N bond of mmen–M2(dobpdc), forming ammonium carbamate. The CO2 binding energies (35.2 to 92.2 kJ/mol) vary with different metal centers. Furthermore, the Bader charge analysis shows that the CO2 molecules acquire 0.42 to 0.47 |e|. This charge is mainly contributed by the mmen, and a small additional amount is from the metal atom bonded with the CO2. The preferred reaction pathway is a two-step reaction. In the first step, the hydrogen bonded complex B changes into an N-coordinated intermediate D with high barriers (0.69 to 1.58 eV). The next step involves the translation and rotation of the chain in the intermediate D, resulting in the formation of the final O-coordinated product I with barriers of 0.22 to 0.61 eV. The higher barriers of CO2 reaction with mmen–M2(dobpdc) relative to attack the primary amine might be due to the larger steric hindrance of mmen. We hope this work will contribute to an improved understanding and development of future amine-grafted materials for efficient CO2 capture.
中文翻译:

N,N'-二甲基乙二胺接枝M 2(dobpdc)中氨基甲酸铵吸收CO 2的形成机理
吸附性能和氨基甲酸酯为CO铵的形成机制2中捕获N,N- '二甲基乙二胺(mmen)接枝的中号2(dobpdc)(dobpdc 4- = 4,4'- dioxidobiphenyl -3,3'-二羧酸酯; M =通过密度泛函理论(DFT)计算研究了Mg,Sc-Zn(除Ni外)。我们看到mmen分子通过M–N键连接到金属位点,并且与相邻的mmen分子具有氢键。mmen的结合能范围为135.4至184.0kJ / mol。CO 2通过插入mmen-M 2(dobpdc)的M-N键中而捕获,形成氨基甲酸铵。一氧化碳2结合能(35.2至92.2 kJ / mol)随金属中心的不同而变化。此外,Bader电荷分析表明,CO 2分子获得0.42至0.47 |。e | 。该电荷主要由mmen贡献,并且少量额外的来自与CO 2键合的金属原子。优选的反应途径是两步反应。在第一步中,氢键配合物B变为具有高势垒(0.69至1.58 eV)的N配位中间体D。下一步涉及链在中间体D中的平移和旋转,从而形成最终的O配位产物I,其势垒为0.22至0.61 eV。mmen–M 2与CO 2反应的较高壁垒(dobpdc)相对于伯胺的攻击可能是由于mmen的较大位阻。我们希望这项工作将有助于增进对未来胺接枝材料的了解和开发,以实现有效的CO 2捕集。
更新日期:2020-11-25
中文翻译:

N,N'-二甲基乙二胺接枝M 2(dobpdc)中氨基甲酸铵吸收CO 2的形成机理
吸附性能和氨基甲酸酯为CO铵的形成机制2中捕获N,N- '二甲基乙二胺(mmen)接枝的中号2(dobpdc)(dobpdc 4- = 4,4'- dioxidobiphenyl -3,3'-二羧酸酯; M =通过密度泛函理论(DFT)计算研究了Mg,Sc-Zn(除Ni外)。我们看到mmen分子通过M–N键连接到金属位点,并且与相邻的mmen分子具有氢键。mmen的结合能范围为135.4至184.0kJ / mol。CO 2通过插入mmen-M 2(dobpdc)的M-N键中而捕获,形成氨基甲酸铵。一氧化碳2结合能(35.2至92.2 kJ / mol)随金属中心的不同而变化。此外,Bader电荷分析表明,CO 2分子获得0.42至0.47 |。e | 。该电荷主要由mmen贡献,并且少量额外的来自与CO 2键合的金属原子。优选的反应途径是两步反应。在第一步中,氢键配合物B变为具有高势垒(0.69至1.58 eV)的N配位中间体D。下一步涉及链在中间体D中的平移和旋转,从而形成最终的O配位产物I,其势垒为0.22至0.61 eV。mmen–M 2与CO 2反应的较高壁垒(dobpdc)相对于伯胺的攻击可能是由于mmen的较大位阻。我们希望这项工作将有助于增进对未来胺接枝材料的了解和开发,以实现有效的CO 2捕集。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号