当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reductive Amination of Biobased Levulinic Acid to Unnatural Chiral γ-Amino Acid Using an Engineered Amine Dehydrogenase
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-11-11 , DOI: 10.1021/acssuschemeng.0c04647 Rui-Feng Cai 1 , Lei Liu 1 , Fei-Fei Chen 1 , Aitao Li 2, 3 , Jian-He Xu 1, 3 , Gao-Wei Zheng 1, 3
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-11-11 , DOI: 10.1021/acssuschemeng.0c04647 Rui-Feng Cai 1 , Lei Liu 1 , Fei-Fei Chen 1 , Aitao Li 2, 3 , Jian-He Xu 1, 3 , Gao-Wei Zheng 1, 3
Affiliation
|
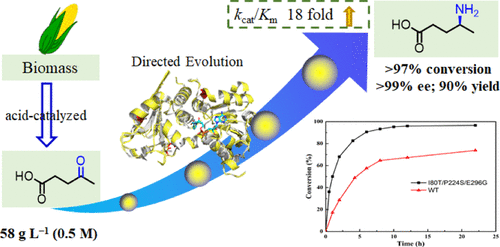
Optically pure (S)-4-aminopentanoic acid is a pivotal precursor in the synthesis of therapeutic molecules and pyrrolidinone derivatives. Enantioselective reductive amination of levulinic acid catalyzed by amine dehydrogenases, a readily sustainable material from biobased lignocellulosic waste, represents an attractive approach for the synthesis of (S)-4-aminopentanoic acid. However, the natural amine dehydrogenases reported so far showed insufficient activity toward levulinic acid. Herein, we engineered a naturally occurring amine dehydrogenase from a thermophilic bacterium Petrotoga mobilis (PmAmDH) by directed evolution. The catalytic efficiency of the most active mutant PmAmDHI80T/P224S/E296G was elevated by 18 folds in comparison to the wild-type enzyme. Using PmAmDHI80T/P224S/E296G coupled with formate dehydrogenase for reduced nicotinamide adenine dinucleotide regeneration, 0.5 M of levulinic acid was reductively aminated in more than 97% conversion at 40 °C, generating the corresponding product (S)-4-aminopentanoic acid with >99% ee and 90% yield. Furthermore, we also successfully developed a chemoenzymatic cascade route for the synthesis of (S)-4-aminopentanoic acid from renewable starch. These results indicated that the engineered amine dehydrogenase PmAmDHI80T/P224S/E296G can serve as an efficient biocatalyst for the manufacture of highly valued chiral unnatural amino acids using renewable feedstocks.
中文翻译:

使用工程胺脱氢酶将生物基亮丙氨酸还原为非天然手性γ-氨基酸
光学纯的(S)-4-氨基戊酸是治疗性分子和吡咯烷酮衍生物合成中的关键前体。由胺基脱氢酶催化的乙酰丙酸的对映选择性还原胺化反应,是一种易于持久的生物基木质纤维素废料,是合成(S)-4-氨基戊酸的一种有吸引力的方法。但是,迄今为止报道的天然胺脱氢酶对乙酰丙酸的活性不足。在这里,我们通过定向进化工程改造了嗜热菌运动发酵单胞菌(Pm AmDH)的天然胺脱氢酶。活性最高的Pm AmDH I80T / P224S / E296G突变体的催化效率与野生型酶相比,其升高了18倍。使用Pm AmDH I80T / P224S / E296G结合甲酸脱氢酶减少烟酰胺腺嘌呤二核苷酸的再生,在40°C时0.5M乙酰丙酸被还原胺化,转化率超过97%,生成相应的产物(S)-4-氨基戊酸ee> 99%,产率90%。此外,我们还成功开发了一种化学酶促级联途径,用于由可再生淀粉合成(S)-4-氨基戊酸。这些结果表明工程胺脱氢酶Pm AmDH I80T / P224S / E296G 可以用作使用可再生原料生产高价值手性非天然氨基酸的有效生物催化剂。
更新日期:2020-11-23
中文翻译:

使用工程胺脱氢酶将生物基亮丙氨酸还原为非天然手性γ-氨基酸
光学纯的(S)-4-氨基戊酸是治疗性分子和吡咯烷酮衍生物合成中的关键前体。由胺基脱氢酶催化的乙酰丙酸的对映选择性还原胺化反应,是一种易于持久的生物基木质纤维素废料,是合成(S)-4-氨基戊酸的一种有吸引力的方法。但是,迄今为止报道的天然胺脱氢酶对乙酰丙酸的活性不足。在这里,我们通过定向进化工程改造了嗜热菌运动发酵单胞菌(Pm AmDH)的天然胺脱氢酶。活性最高的Pm AmDH I80T / P224S / E296G突变体的催化效率与野生型酶相比,其升高了18倍。使用Pm AmDH I80T / P224S / E296G结合甲酸脱氢酶减少烟酰胺腺嘌呤二核苷酸的再生,在40°C时0.5M乙酰丙酸被还原胺化,转化率超过97%,生成相应的产物(S)-4-氨基戊酸ee> 99%,产率90%。此外,我们还成功开发了一种化学酶促级联途径,用于由可再生淀粉合成(S)-4-氨基戊酸。这些结果表明工程胺脱氢酶Pm AmDH I80T / P224S / E296G 可以用作使用可再生原料生产高价值手性非天然氨基酸的有效生物催化剂。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号