Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High Intensity Focused Ultrasound-Responsive and Ultrastable Cerasomal Perfluorocarbon Nanodroplets for Alleviating Tumor Multidrug Resistance and Epithelial–Mesenchymal Transition
ACS Nano ( IF 15.8 ) Pub Date : 2020-11-11 , DOI: 10.1021/acsnano.0c07287
Xiaotu Ma 1, 2, 3 , Meinan Yao 4 , Jiyun Shi 2 , Xiaoda Li 5 , Yu Gao 2 , Qi Luo 2, 6 , Rui Hou 4 , Xiaolong Liang 1 , Fan Wang 2, 4, 6
ACS Nano ( IF 15.8 ) Pub Date : 2020-11-11 , DOI: 10.1021/acsnano.0c07287
Xiaotu Ma 1, 2, 3 , Meinan Yao 4 , Jiyun Shi 2 , Xiaoda Li 5 , Yu Gao 2 , Qi Luo 2, 6 , Rui Hou 4 , Xiaolong Liang 1 , Fan Wang 2, 4, 6
Affiliation
|
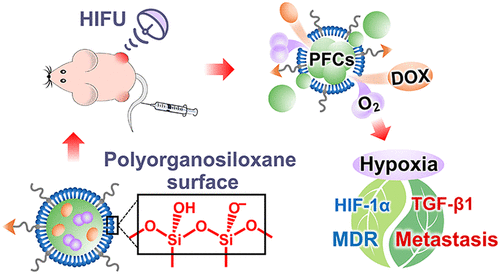
Hypoxia is a hostile hallmark of most solid tumors, which often leads to multidrug resistance (MDR) and causes the failure of chemotherapy. Hypoxia also promotes epithelial–mesenchymal transition (EMT), leading to acceleration of tumor metastasis. Many chemotherapeutic drugs can further exacerbate hypoxia and thus promote metastasis. Therefore, relieving hypoxia is necessary for chemotherapy to inhibit both MDR and EMT. Herein, highly stable cerasomal perfluorocarbon nanodroplets with an atomic layer of polyorganosiloxane surface and pH-sensitive tumor-targeting peptide (D-vPCs-O2) were fabricated to co-deliver oxygen and therapeutic drug, doxorubicin. High-intensity focused ultrasound (HIFU) was utilized to trigger the co-release of doxorubicin and oxygen and simultaneously enhance ultrasound imaging, therefore achieving imaging-guided drug delivery. Mild-temperature HIFU (M-HIFU) not only triggered oxygen release from nanodroplets but also slightly elevated tumor temperature to accelerate tumor blood flow. The oxygen release and temperature elevation jointly relieved tumor hypoxia and alleviated MDR, which greatly enhanced the drug therapeutic efficacy as compared to clinically used doxorubicin and Doxil. Overall side effects were also largely reduced owing to the ultrastable drug loading of cerasome. The improvement of insufficient chemotherapy and the relief of tumor hypoxia corporately down-regulated TGF-β1, leading to the alleviation of EMT, and therefore significantly inhibited tumor metastasis. When “D-vPCs-O2 + M-HIFU” was utilized as a neoadjuvant chemotherapy, nanodroplets down-regulated heat shock proteins, reducing tumor relapse after the high-temperature HIFU (H-HIFU)-mediated hyperthermia ablation. The chemo-hyperthermia therapy totally eradicated tumors without any relapse or metastasis, providing a promising way to treat the triple-negative breast cancer, which is highly malignant, easily metastatic, and lacks effective treatments.
中文翻译:

高强度聚焦超声响应性和超稳定的陶瓷全氟碳纳米液滴可减轻肿瘤的多药耐药性和上皮-间质转化
缺氧是大多数实体瘤的敌对标志,通常导致多药耐药(MDR)并导致化疗失败。缺氧还促进上皮-间质转化(EMT),导致肿瘤转移加速。许多化疗药物可进一步加剧缺氧,从而促进转移。因此,缓解缺氧对于化疗抑制MDR和EMT都是必要的。本文中,具有聚有机硅氧烷表面原子层和pH敏感肿瘤靶向肽(D-vPCs-O 2)可以共同输送氧气和治疗药物阿霉素。利用高强度聚焦超声(HIFU)触发阿霉素和氧气的共释放并同时增强超声成像,因此实现了成像引导下的药物输送。温和的HIFU(M-HIFU)不仅触发了纳米液滴中的氧气释放,而且使肿瘤温度略微升高,从而加速了肿瘤的血流。氧气释放和温度升高共同缓解了肿瘤的缺氧,减轻了MDR,与临床使用的阿霉素和多西尔相比,大大提高了药物的治疗效果。由于血清中超稳定的药物负载,总体副作用也大大降低。化疗不足的改善和肿瘤缺氧的缓解,共同降低了TGF-β1,导致EMT的减轻,因此显着抑制了肿瘤转移。当“ D-vPCs-O“ 2 + M-HIFU”被用作新辅助化疗,纳米液滴下调了热休克蛋白,减少了高温HIFU(H-HIFU)介导的高温消融后的肿瘤复发。化学高热疗法可完全消灭肿瘤,而无任何复发或转移,这为治疗三阴性阴性乳腺癌提供了一种有前途的方法,这种乳腺癌具有高度恶性,易转移且缺乏有效的治疗方法。
更新日期:2020-11-25
中文翻译:

高强度聚焦超声响应性和超稳定的陶瓷全氟碳纳米液滴可减轻肿瘤的多药耐药性和上皮-间质转化
缺氧是大多数实体瘤的敌对标志,通常导致多药耐药(MDR)并导致化疗失败。缺氧还促进上皮-间质转化(EMT),导致肿瘤转移加速。许多化疗药物可进一步加剧缺氧,从而促进转移。因此,缓解缺氧对于化疗抑制MDR和EMT都是必要的。本文中,具有聚有机硅氧烷表面原子层和pH敏感肿瘤靶向肽(D-vPCs-O 2)可以共同输送氧气和治疗药物阿霉素。利用高强度聚焦超声(HIFU)触发阿霉素和氧气的共释放并同时增强超声成像,因此实现了成像引导下的药物输送。温和的HIFU(M-HIFU)不仅触发了纳米液滴中的氧气释放,而且使肿瘤温度略微升高,从而加速了肿瘤的血流。氧气释放和温度升高共同缓解了肿瘤的缺氧,减轻了MDR,与临床使用的阿霉素和多西尔相比,大大提高了药物的治疗效果。由于血清中超稳定的药物负载,总体副作用也大大降低。化疗不足的改善和肿瘤缺氧的缓解,共同降低了TGF-β1,导致EMT的减轻,因此显着抑制了肿瘤转移。当“ D-vPCs-O“ 2 + M-HIFU”被用作新辅助化疗,纳米液滴下调了热休克蛋白,减少了高温HIFU(H-HIFU)介导的高温消融后的肿瘤复发。化学高热疗法可完全消灭肿瘤,而无任何复发或转移,这为治疗三阴性阴性乳腺癌提供了一种有前途的方法,这种乳腺癌具有高度恶性,易转移且缺乏有效的治疗方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号