当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Indol‐2‐ylidene (IdY): Ambiphilic N‐Heterocyclic Carbene Derived from Indole**
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-11-09 , DOI: 10.1002/chem.202004879
Hyunho Kim 1 , Minseop Kim 1 , Hayoung Song 1 , Eunsung Lee 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-11-09 , DOI: 10.1002/chem.202004879
Hyunho Kim 1 , Minseop Kim 1 , Hayoung Song 1 , Eunsung Lee 1
Affiliation
|
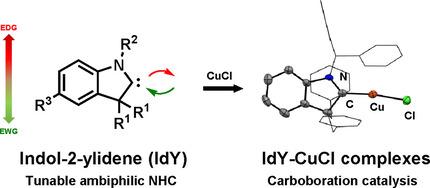
The synthesis of ambiphilic N‐heterocyclic carbene ligand, indol‐2‐ylidene (IdY, A), is described. A series of indolenium precursors (2 a–f) were prepared on a gram scale in good yields. Trapping experiments with elemental selenium, [RhCl(cod)]2 and CuCl provided the expected carbene adducts. Further computational and spectroscopic studies supported the ambiphilicity of IdY, which lies between cyclic (alkyl)(amino)carbenes (CAAC‐5) and cyclic (amino)(aryl)carbene (CAArC). The copper complexes (6) show high percent buried volume (% Vbur = 58.1) and allow for carboboration of terminal alkynes within 30 minutes in a demonstration of synthetic utility with good yields and high regioselectivity.
中文翻译:

吲哚-2-亚烷基(IdY):源自吲哚的双亲N-杂环卡宾**
描述了双歧性N-杂环卡宾配体吲哚-2-亚烷基(IdY,A)的合成。以克为单位制备了一系列吲哚前体(2 a - f),收率很高。用元素硒,[RhCl(cod)] 2和CuCl进行的捕集实验提供了预期的卡宾加合物。进一步的计算和光谱研究支持了IdY的歧义性,它位于环状(烷基)(氨基)卡宾(CAAC-5)和环状(氨基)(芳基)卡宾(CAArC)之间。铜络合物(6)的掩埋体积百分比高(% V bur = 58.1),并允许在30分钟内对末端炔烃进行碳硼化,以证明合成实用性高收率和高区域选择性。
更新日期:2020-11-09
中文翻译:

吲哚-2-亚烷基(IdY):源自吲哚的双亲N-杂环卡宾**
描述了双歧性N-杂环卡宾配体吲哚-2-亚烷基(IdY,A)的合成。以克为单位制备了一系列吲哚前体(2 a - f),收率很高。用元素硒,[RhCl(cod)] 2和CuCl进行的捕集实验提供了预期的卡宾加合物。进一步的计算和光谱研究支持了IdY的歧义性,它位于环状(烷基)(氨基)卡宾(CAAC-5)和环状(氨基)(芳基)卡宾(CAArC)之间。铜络合物(6)的掩埋体积百分比高(% V bur = 58.1),并允许在30分钟内对末端炔烃进行碳硼化,以证明合成实用性高收率和高区域选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号