当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Atropisomeric Biaryls using Biaryl 2,5‐Diphenylphospholanes as Ligands for Palladium‐Catalysed Suzuki‐Miyaura Reactions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-08 , DOI: 10.1002/adsc.202001211 Liam Byrne 1 , Christian Sköld 2 , Per‐Ola Norrby 3 , Rachel H. Munday 4 , Andrew R. Turner 1 , Peter D. Smith 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-08 , DOI: 10.1002/adsc.202001211 Liam Byrne 1 , Christian Sköld 2 , Per‐Ola Norrby 3 , Rachel H. Munday 4 , Andrew R. Turner 1 , Peter D. Smith 1
Affiliation
|
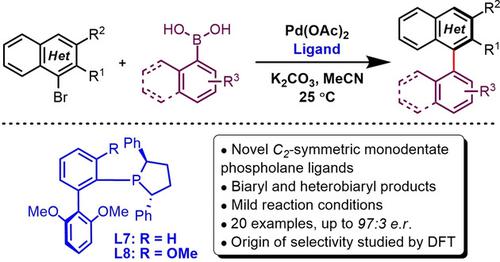
Here we describe the development of biaryl 2,5‐diphenylphospholanes as a new class of C2‐symmetric, monodentate ligands for asymmetric Suzuki‐Miyaura (ASM) reactions. Screening of a series of exemplary phospholanes led to the identification of two ligands that were used to prepare a range of atropisomeric biaryl and heterobiaryl products with good to excellent levels of enantioselectivity (up to 97:3 e.r.) under mild conditions. DFT studies suggest that the formation of a constraining ligand pocket and coordination of one of the biaryl methoxy groups in the optimised ligands to the metal centre is crucial for restricting conformational freedom in the bond‐forming step.
中文翻译:

以联芳基2,5-二苯基膦为配体,对钯催化的Suzuki-Miyaura反应进行对映异构联芳基的对映选择性合成
在这里,我们将联芳基2,5-二苯基膦酸酯的开发描述为用于不对称铃木-宫浦(ASM)反应的一类新的C 2对称单齿配体。一系列示例性膦酸酯的筛选导致鉴定了两个配体,这些配体用于在温和条件下制备对映异构性联芳基和杂联二芳基产品,对映选择性好至极好(至高97:3 er)。DFT研究表明,约束配位体的形成以及优化配体中的联芳基甲氧基中的一个与金属中心的配位对于限制键形成步骤中的构象自由度至关重要。
更新日期:2021-01-05
中文翻译:

以联芳基2,5-二苯基膦为配体,对钯催化的Suzuki-Miyaura反应进行对映异构联芳基的对映选择性合成
在这里,我们将联芳基2,5-二苯基膦酸酯的开发描述为用于不对称铃木-宫浦(ASM)反应的一类新的C 2对称单齿配体。一系列示例性膦酸酯的筛选导致鉴定了两个配体,这些配体用于在温和条件下制备对映异构性联芳基和杂联二芳基产品,对映选择性好至极好(至高97:3 er)。DFT研究表明,约束配位体的形成以及优化配体中的联芳基甲氧基中的一个与金属中心的配位对于限制键形成步骤中的构象自由度至关重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号