Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-07 , DOI: 10.1016/j.tet.2020.131721 Vakhid A. Mamedov , Nataliya A. Zhukova , Victor V. Syakaev , Aidar T. Gubaidullin , Milyausha S. Kadyrova , Tat’yana N. Beschastnova , Il′dar Kh. Rizvanov , Shamil K. Latypov
|
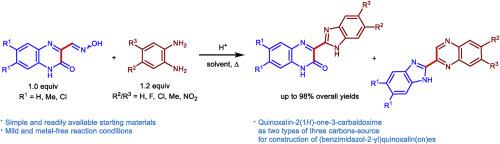
Interaction of quinoxalin-2(1H)-one-3-carbaldoximes with 1,2-benzenediamine derivatives in AcOH or n-BuOH at reflux in the presence of sulfuric acid as catalyst was found to give 3-(benzimidazol-2-yl)quinoxalin-2(1H)-ones and 2-(benzimidazol-2-yl)quinoxalines in relatively good yields, which formed as a result of the Weidenhagen reaction and Mamedov rearrangement, respectively. The reaction in AcOH afforded 2-(benzimidazol-2-yl)quinoxalines in preference to the 3-(benzimidazol-2-yl)quinoxalin-2(1H)-ones, while the reverse result was obtained in n-BuOH. The synthetic utility of this strategy was illustrated by the concise, one-pot synthesis of 3-(3H-imidazo[4,5-b]pyridin-2-yl)quinoxalin-2(1H)-ones.
中文翻译:

3-(苯并咪唑-2-基)的同时形成喹喔啉-2(1 H ^) -酮和2-(苯并咪唑-2-基)从喹喔啉-2-喹喔啉(1 ħ) -酮-3- carbaldoximes当暴露于1 ,2-苯二胺
在硫酸为催化剂的条件下,回流下喹喔啉-2(1 H)-一-3-羰醛肟与1,2-苯二胺衍生物在AcOH或n- BuOH中的相互作用,得到3-(苯并咪唑-2-基)喹喔啉-2(1 H)-酮和2-(苯并咪唑-2-基)喹喔啉的收率相对较高,这分别是由于Weidenhagen反应和Mamedov重排而形成的。在AcOH中的反应提供的2-(苯并咪唑-2-基)喹喔啉优先于3-(苯并咪唑-2-基)喹喔啉-2(1 H)-酮,而在n- BuOH中得到相反的结果。简明的一锅合成3-(3 H-咪唑并[4,5- b] pyridin-2-yl)quinoxalin-2(1 H)-ones。













































 京公网安备 11010802027423号
京公网安备 11010802027423号