European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-11-07 , DOI: 10.1016/j.ejmech.2020.113003 N. Perin , L. Hok , A. Beč , L. Persoons , E. Vanstreels , D. Daelemans , R. Vianello , M. Hranjec
|
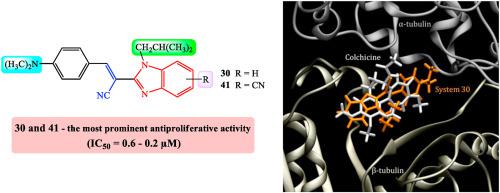
We present the design, synthesis and biological activity of novel N-substituted benzimidazole based acrylonitriles as potential inhibitors of the tubulin polymerization. Their synthesis was achieved using classical linear organic and microwave assisted techniques, starting from aromatic aldehydes and N-substituted-2-cyanomethylbenzimidazoles. All newly prepared compounds were tested for their antiproliferative activity in vitro on eight human cancer cell lines and one reference non-cancerous assay. N,N-dimethylamino substituted acrylonitriles 30 and 41, bearing N-isobutyl and cyano substituents placed on the benzimidazole nuclei, showed strong and selective antiproliferative activity in the submicromolar range of inhibitory concentrations (IC50 0.2 – 0.6 μM), while being significantly less toxic than reference systems docetaxel and staurosporine, thus promoting them as lead compounds. Mechanism of action studies demonstrated that two most active compounds inhibited the polymerization of tubulin. Computational analysis confirmed the suitability of the employed benzimidazole-acrylonitrile skeleton for the binding within the colchicine binding site in tubulin, thus rationalizing the observed antitumor activities, and demonstrated that E-isomers are active substances. It also provided structural determinants affecting both the binding position and the matching affinities, identifying the attached NMe2 group as the most dominant in promoting the binding, which allows ligands to optimize favorable cation∙∙∙π and hydrogen bonding interactions with Lys352.
中文翻译:

N-取代的苯并咪唑丙烯腈作为体外微管蛋白聚合抑制剂:合成,生物学活性和计算分析
我们提出了新型的N-取代的苯并咪唑基丙烯腈作为微管蛋白聚合的潜在抑制剂的设计,合成和生物活性。它们的合成是使用经典的线性有机和微波辅助技术完成的,从芳香醛和N-取代的2-氰基甲基苯并咪唑类化合物开始。所有新制备的化合物均在八种人类癌细胞系中进行了体外抗增殖活性的测试,并在一项非癌性参考测定中进行了测试。N,N-二甲基氨基取代的丙烯腈30和41,含N苯并咪唑核上的-异丁基和氰基取代基在亚微摩尔浓度的抑制浓度下(IC 50 0.2 – 0.6μM)显示出强而有选择性的抗增殖活性,但毒性明显低于参考系统多西他赛和星形孢菌素,因此促进它们作为铅化合物。作用机理研究表明,两种活性最高的化合物抑制微管蛋白的聚合。计算分析证实所用的苯并咪唑-丙烯腈骨架适合于微管蛋白中秋水仙碱结合位点内的结合,从而使观察到的抗肿瘤活性合理化,并证明了E-异构体是活性物质。它还提供了影响结合位置和匹配亲和力的结构决定因素,确定了附着的NMe 2基团是促进结合的最主要因素,这使配体能够优化与Lys352的有利阳离子∙∙∙π和氢键相互作用。







































 京公网安备 11010802027423号
京公网安备 11010802027423号