当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral–Achiral Ligand Synergy in Enhancing the Chiroptical Activity of Diphosphine-Protected Au13 Clusters
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.jpcc.0c07064 Yasuhiko Sato 1 , Masaki Mitani 1 , Hiroshi Yao 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.jpcc.0c07064 Yasuhiko Sato 1 , Masaki Mitani 1 , Hiroshi Yao 1
Affiliation
|
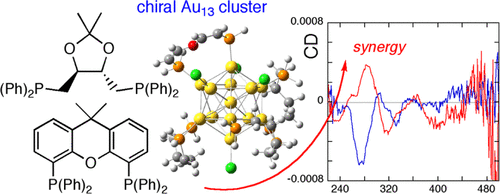
We report chiroptical activity in Au13 clusters protected by binary mixed ligands of chiral and achiral diphosphines. S,S-DIOP is used as the chiral diphosphine, and the achiral diphosphine is Xantphos, Dppe, or Dppbz, where S,S-DIOP, Xantphos, Dppe, and Dppbz stand for (4S,5S)-4,5-bis(diphenylphosphinomethyl)-2,2-dimethyl-1,3-dioxolane, 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene, 1,2-bis(diphenylphosphino)ethane, and 1,2-bis(diphenylphosphino)benzene, respectively. The initial feed molar ratio of chiral/achiral ligands is 1/0, 3/1, or 1/1 in the synthesis. Remarkably, the Au13 cluster synthesized at S,S-DIOP/Xantphos = 3/1 ratio exhibits high chiroptical activity or anisotropy factor than that protected by pure S,S-DIOP in a core-based transition region (≤∼4 eV). To account for this chiral–achiral ligand synergy effect, quantum chemical calculations and geometrical quantifications based on the Hausdorff chirality measure (HCM) are performed for model Au13 clusters, giving strong suggestions that the observed chiroptical enhancement can be due both to the effect of limited isomer configurations and larger HCM values compared to that of the Au13 cluster passivated by pure chiral diphosphine. We believe that the present cluster system with chiral–achiral ligand synergy will be a fascinating model for further understanding the cluster structure–chiroptical activity relationships.
中文翻译:

手性-非手性配体协同作用,增强二膦保护的Au 13团簇的手性活性
我们报告了由手性和非手性二膦的二元混合配体保护的Au 13簇中的按摩活性。S,S -DIOP用作手性二膦,非手性二膦是Xantphos,Dppe或Dppbz,其中S,S -DIOP,Xantphos,Dppe和Dppbz代表(4 S,5 S)-4,5 -双(二苯基膦基甲基)-2,2-二甲基-1,3-二氧戊环,4,5-双(二苯基膦基)-9,9-二甲基x吨,1,2-双(二苯基膦基)乙烷和1,2-双(二苯基膦基)苯。在合成中手性/非手性配体的初始进料摩尔比为1 / 0、3 / 1或1/1。值得注意的是,Au 13团簇在S,S -DIOP / Xantphos = 3/1的比例在基于核的过渡区(≤〜4 eV)中表现出比纯S,S -DIOP更高的手性或各向异性因子。为了说明这种手性-非手性配体协同作用,对Au 13团簇进行了基于Hausdorff手性测度(HCM)的量子化学计算和几何定量,强烈建议观察到的手性增强可能是由于与Au 13相比,异构体构型有限,HCM值更大簇被纯手性二膦钝化。我们相信,具有手性-非手性配体协同作用的当前簇系统将是进一步理解簇结构与手性活动关系的一个引人入胜的模型。
更新日期:2020-11-19
中文翻译:

手性-非手性配体协同作用,增强二膦保护的Au 13团簇的手性活性
我们报告了由手性和非手性二膦的二元混合配体保护的Au 13簇中的按摩活性。S,S -DIOP用作手性二膦,非手性二膦是Xantphos,Dppe或Dppbz,其中S,S -DIOP,Xantphos,Dppe和Dppbz代表(4 S,5 S)-4,5 -双(二苯基膦基甲基)-2,2-二甲基-1,3-二氧戊环,4,5-双(二苯基膦基)-9,9-二甲基x吨,1,2-双(二苯基膦基)乙烷和1,2-双(二苯基膦基)苯。在合成中手性/非手性配体的初始进料摩尔比为1 / 0、3 / 1或1/1。值得注意的是,Au 13团簇在S,S -DIOP / Xantphos = 3/1的比例在基于核的过渡区(≤〜4 eV)中表现出比纯S,S -DIOP更高的手性或各向异性因子。为了说明这种手性-非手性配体协同作用,对Au 13团簇进行了基于Hausdorff手性测度(HCM)的量子化学计算和几何定量,强烈建议观察到的手性增强可能是由于与Au 13相比,异构体构型有限,HCM值更大簇被纯手性二膦钝化。我们相信,具有手性-非手性配体协同作用的当前簇系统将是进一步理解簇结构与手性活动关系的一个引人入胜的模型。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号