当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrazine Formation via Coupling of a Nickel(III)–NH2 Radical
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-05 , DOI: 10.1002/anie.202013119 Nina X Gu 1 , Paul H Oyala 1 , Jonas C Peters 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-05 , DOI: 10.1002/anie.202013119 Nina X Gu 1 , Paul H Oyala 1 , Jonas C Peters 1
Affiliation
|
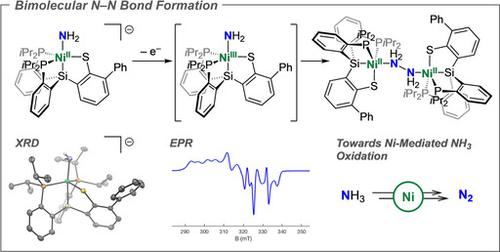
M(NHx) intermediates involved in N−N bond formation are central to ammonia oxidation (AO) catalysis, an enabling technology to ultimately exploit ammonia (NH3) as an alternative fuel source. While homocoupling of a terminal amide species (M‐NH2) to form hydrazine (N2H4) has been proposed, well‐defined examples are without precedent. Herein, we discuss the generation and electronic structure of a NiIII‐NH2 species that undergoes bimolecular coupling to generate a NiII2(N2H4) complex. This hydrazine adduct can be further oxidized to a structurally unusual Ni2(N2H2) species; this releases N2 in the presence of NH3, thus establishing a synthetic cycle for Ni‐mediated AO. Distribution of the redox load for H2N‐NH2 formation via NH2 coupling between two metal centers presents an attractive strategy for AO catalysis using Earth‐abundant, late first‐row metals.
中文翻译:

通过镍 (III)-NH2 自由基偶联形成肼
参与 N−N 键形成的 M(NH x ) 中间体是氨氧化 (AO) 催化的核心,这是一种最终利用氨 (NH 3 ) 作为替代燃料来源的技术。虽然已经提出了末端酰胺物质 (M-NH 2 ) 的自偶联形成肼 (N 2 H 4 ),但明确的例子是没有先例的。在此,我们讨论了 Ni III ‐NH 2物质的生成和电子结构,该物质经过双分子偶联生成 Ni II 2 (N 2 H 4 ) 络合物。这种肼加合物可以进一步氧化成结构上不寻常的 Ni 2 (N 2 H 2 ) 物质;这在 NH 3存在的情况下释放 N 2 ,从而建立 Ni 介导的 AO 的合成循环。通过两个金属中心之间的 NH 2耦合形成 H 2 N-NH 2的氧化还原负荷的分布为使用地球丰富的晚期第一行金属进行 AO 催化提供了一种有吸引力的策略。
更新日期:2020-11-05
中文翻译:

通过镍 (III)-NH2 自由基偶联形成肼
参与 N−N 键形成的 M(NH x ) 中间体是氨氧化 (AO) 催化的核心,这是一种最终利用氨 (NH 3 ) 作为替代燃料来源的技术。虽然已经提出了末端酰胺物质 (M-NH 2 ) 的自偶联形成肼 (N 2 H 4 ),但明确的例子是没有先例的。在此,我们讨论了 Ni III ‐NH 2物质的生成和电子结构,该物质经过双分子偶联生成 Ni II 2 (N 2 H 4 ) 络合物。这种肼加合物可以进一步氧化成结构上不寻常的 Ni 2 (N 2 H 2 ) 物质;这在 NH 3存在的情况下释放 N 2 ,从而建立 Ni 介导的 AO 的合成循环。通过两个金属中心之间的 NH 2耦合形成 H 2 N-NH 2的氧化还原负荷的分布为使用地球丰富的晚期第一行金属进行 AO 催化提供了一种有吸引力的策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号