当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Step Synthesis of Nanostructured Cu–Mn/TiO2 via Flame Spray Pyrolysis: Application to Catalytic Combustion of CO and CH4
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-11-03 , DOI: 10.1021/acs.energyfuels.0c02747 Xing Yuan 1 , Menglei Qing 1 , Lingquan Meng 1 , Haibo Zhao 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-11-03 , DOI: 10.1021/acs.energyfuels.0c02747 Xing Yuan 1 , Menglei Qing 1 , Lingquan Meng 1 , Haibo Zhao 1
Affiliation
|
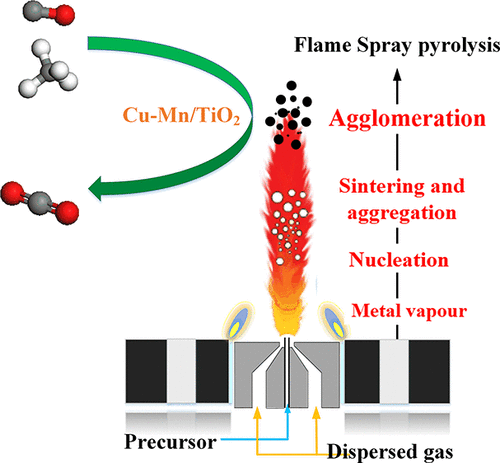
Catalytic combustion has been widely applied to remove the trace combustible pollutants. However, the earth-abundant and high-performance nanocatalysts are still the main research focus on promoting catalytic efficiency. Herein, the Cu and Mn mixed oxides supported on TiO2 nanoparticles with various Cu and Mn molar contents synthesized via the flame spray pyrolysis (FSP) technique are utilized in the catalytic oxidation of lean CO and CH4. Initially, the Cu–Mn/TiO2 nanocatalysts are composed of spherical structures with a diameter of about 20 nm, whose specific surface area is between 60 and 90 m2/g. The Cu element is more evenly distributed on the TiO2 surface than the Mn element, owing to the distinctly different ion radii. Both the copper and manganese cations could incorporate into the TiO2 lattice, which generates oxygen vacancies and enhances the diffusion of oxygen ions, causing the transformation of the antanse to rutile phase. When the molar content of the Cu–Mn increases to less than 30 mol %, the temperature of its reduction peak keeps decreasing due to the hydrogen spillover effect. Moreover, the catalytic performances of the Cu–Mn/TiO2 with 12 mol % loading (12CMT) are all optimal during the low-temperature and the high-temperature stages, which are superior to the FSP-made copper manganese or copper titanium oxides. This is attributed to the small crystal particles, highly dispersed active components of CuOx and MnOx, and the higher ratios of Cu1+/Cu and Mn4+–Oads Lewis acid–base pairs. In addition, the strong interaction between Cu–Mn components and rutile phase support can tremendously promote the activity of catalytic combustion. Under the simulated flue gas, the catalytic properties of 12CMT decreases in comparison with those of CO and CH4 mixed gas due to the introduction of CO2. Ultimately, the Cu–Mn/TiO2 samples exhibit the outstanding water resistance, thanks to the hydrophobization of the catalyst surface.
中文翻译:

火焰喷雾热解一步合成纳米结构Cu-Mn / TiO 2:在CO和CH 4催化燃烧中的应用
催化燃烧已广泛应用于去除痕量可燃污染物。然而,富含地球和高性能的纳米催化剂仍然是提高催化效率的主要研究重点。在本文中,通过火焰喷雾热解(FSP)技术合成的,具有不同的铜和锰摩尔含量的负载在TiO 2纳米颗粒上的Cu和Mn混合氧化物被用于贫CO和CH 4的催化氧化。最初,Cu–Mn / TiO 2纳米催化剂由直径约20 nm的球形结构组成,其比表面积在60至90 m 2 / g之间。Cu元素更均匀地分布在TiO 2上由于离子半径截然不同,因此表面比Mn元素高。铜和锰阳离子都可以结合到TiO 2晶格中,从而产生氧空位并增强氧离子的扩散,从而导致花椰菜酸转变为金红石相。当Cu-Mn的摩尔含量增加到小于30 mol%时,由于氢溢出效应,其还原峰的温度持续降低。此外,在低温和高温阶段,负载量为12 mol%的Cu–Mn / TiO 2(12CMT)的催化性能均最佳,优于FSP制得的铜锰或铜钛氧化物。这归因于小的晶体颗粒,高度分散的CuO活性成分X和MnO X,和Cu的比率较高1+ /铜,锰4+ -O广告路易斯酸碱对。此外,铜锰成分与金红石相载体之间的强相互作用可以极大地促进催化燃烧的活性。在模拟烟气条件下,由于引入了CO 2,与CO和CH 4混合气体相比,12CMT的催化性能下降。归根结底,由于催化剂表面的疏水作用,Cu–Mn / TiO 2样品表现出出色的耐水性。
更新日期:2020-11-19
中文翻译:

火焰喷雾热解一步合成纳米结构Cu-Mn / TiO 2:在CO和CH 4催化燃烧中的应用
催化燃烧已广泛应用于去除痕量可燃污染物。然而,富含地球和高性能的纳米催化剂仍然是提高催化效率的主要研究重点。在本文中,通过火焰喷雾热解(FSP)技术合成的,具有不同的铜和锰摩尔含量的负载在TiO 2纳米颗粒上的Cu和Mn混合氧化物被用于贫CO和CH 4的催化氧化。最初,Cu–Mn / TiO 2纳米催化剂由直径约20 nm的球形结构组成,其比表面积在60至90 m 2 / g之间。Cu元素更均匀地分布在TiO 2上由于离子半径截然不同,因此表面比Mn元素高。铜和锰阳离子都可以结合到TiO 2晶格中,从而产生氧空位并增强氧离子的扩散,从而导致花椰菜酸转变为金红石相。当Cu-Mn的摩尔含量增加到小于30 mol%时,由于氢溢出效应,其还原峰的温度持续降低。此外,在低温和高温阶段,负载量为12 mol%的Cu–Mn / TiO 2(12CMT)的催化性能均最佳,优于FSP制得的铜锰或铜钛氧化物。这归因于小的晶体颗粒,高度分散的CuO活性成分X和MnO X,和Cu的比率较高1+ /铜,锰4+ -O广告路易斯酸碱对。此外,铜锰成分与金红石相载体之间的强相互作用可以极大地促进催化燃烧的活性。在模拟烟气条件下,由于引入了CO 2,与CO和CH 4混合气体相比,12CMT的催化性能下降。归根结底,由于催化剂表面的疏水作用,Cu–Mn / TiO 2样品表现出出色的耐水性。































 京公网安备 11010802027423号
京公网安备 11010802027423号