当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A case of chain propagation: α-aminoalkyl radicals as initiators for aryl radical chemistry
Chemical Science ( IF 7.6 ) Pub Date : 2020-10-20 , DOI: 10.1039/d0sc04387g Timothée Constantin 1, 2, 3, 4 , Fabio Juliá 1, 2, 3, 4 , Nadeem S. Sheikh 1, 5, 6, 7 , Daniele Leonori 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2020-10-20 , DOI: 10.1039/d0sc04387g Timothée Constantin 1, 2, 3, 4 , Fabio Juliá 1, 2, 3, 4 , Nadeem S. Sheikh 1, 5, 6, 7 , Daniele Leonori 1, 2, 3, 4
Affiliation
|
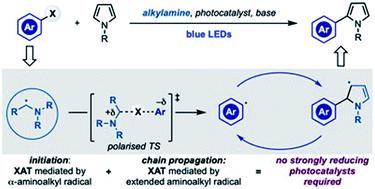
The generation of aryl radicals from the corresponding halides by redox chemistry is generally considered a difficult task due to their highly negative reduction potentials. Here we demonstrate that α-aminoalkyl radicals can be used as both initiators and chain-carriers for the radical coupling of aryl halides with pyrrole derivatives, a transformation often employed to evaluate new highly reducing photocatalysts. This mode of reactivity obviates for the use of strong reducing species and was also competent in the formation of sp2 C–P bonds. Mechanistic studies have delineated some of the key features operating that trigger aryl radical generation and also propagate the chain process.
中文翻译:

链增长的情况:α-氨基烷基自由基作为芳基自由基化学的引发剂
通过氧化还原化学法从相应的卤化物产生芳基通常被认为是一项艰巨的任务,因为它们的还原电位很高。在这里,我们证明了α-氨基烷基自由基既可以用作引发剂,也可以用作链载体,用于芳基卤化物与吡咯衍生物的自由基偶联,这种转化通常用于评估新型的高度还原性光催化剂。这种反应方式避免了使用强还原性物质,并且还可以形成sp 2 C–P键。机理研究描述了触发芳基自由基生成并传播链过程的一些关键特征。
更新日期:2020-11-03
中文翻译:

链增长的情况:α-氨基烷基自由基作为芳基自由基化学的引发剂
通过氧化还原化学法从相应的卤化物产生芳基通常被认为是一项艰巨的任务,因为它们的还原电位很高。在这里,我们证明了α-氨基烷基自由基既可以用作引发剂,也可以用作链载体,用于芳基卤化物与吡咯衍生物的自由基偶联,这种转化通常用于评估新型的高度还原性光催化剂。这种反应方式避免了使用强还原性物质,并且还可以形成sp 2 C–P键。机理研究描述了触发芳基自由基生成并传播链过程的一些关键特征。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号