iScience ( IF 4.6 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.isci.2020.101750 Hoda Safari Yazd , Yu Yang , Long Li , Lu Yang , Xiaowei Li , Xiaoshu Pan , Zhuo Chen , Jianhui Jiang , Cheng Cui , Weihong Tan
|
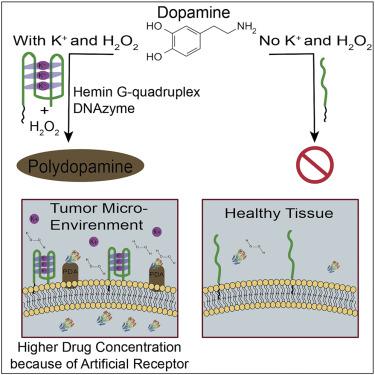
Compared to conventional chemotherapy and radiotherapy, targeted molecular therapy, e.g., antibody-drug conjugates or aptamer-drug conjugates, can specifically identify overexpressed natural receptors on the cancer cell, perform targeted release of anticancer drugs, and achieve targeted killing of tumor cells. However, many natural receptors are also expressed on non-cancer cells, thereby diverting the targeting molecules to healthy cells. By generating artificial cell surface receptors specific to diseased cells, aptamer-drug conjugates can identify these artificial receptors, improve therapeutic efficacy, and decrease the minimum effective dosage. In this study, we use high K+ and high H2O2 of the tumor microenvironment (TME) to produce polydopamine only on living cancer cell membrane. Owing to the significant reactivity of polydopamine with amino groups, e.g., the amino group of proteins, polydopamine can deposit on tumor cells and act as “artificial receptors” for targeted delivery of anticancer drugs with amino groups, in other words, amino-containing drugs and protein drugs.
中文翻译:

聚多巴胺在癌细胞膜上的精确沉积作为靶向药物递送的人工受体
与常规化学疗法和放射疗法相比,靶向分子疗法,例如抗体-药物偶联物或适体-药物偶联物,可以特异性地识别癌细胞上过表达的天然受体,进行靶向释放的抗癌药物,并达到靶向杀死肿瘤细胞的目的。然而,许多天然受体也在非癌细胞上表达,从而将靶向分子转移到健康细胞上。通过产生特定于患病细胞的人工细胞表面受体,适体-药物缀合物可以识别这些人工受体,提高治疗功效并降低最小有效剂量。在这项研究中,我们使用肿瘤微环境(TME)的高K +和高H2O2仅在活癌细胞膜上产生聚多巴胺。































 京公网安备 11010802027423号
京公网安备 11010802027423号