Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-11-02 , DOI: 10.1016/j.freeradbiomed.2020.10.323 Shuangwen Li 1 , Lisi Zheng 1 , Jun Zhang 1 , Xuejun Liu 2 , Zhongming Wu 1
|
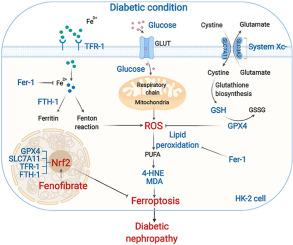
Diabetic nephropathy (DN) is now considered the leading cause of end-stage renal disease. In diabetes, the accumulation of reactive oxygen species (ROS) and iron overload are important determinants that promote the occurrence of DN. However, the underlying mechanism of how they cause diabetic kidney damage remains unclear. Ferroptosis, characterized by iron-dependent lipid peroxidation, provided us with a new idea to explore the progression of DN. Iron overload, reduced antioxidant capability, massive ROS and lipid peroxidation were detected in the kidneys of streptozotocin-induced DBA/2J diabetic mice and high-glucose cultured human renal proximal tubular (HK-2) cells, which were the symbolic changes of ferroptosis. Furthermore, the characteristic mitochondrial morphological changes of ferroptosis were observed in high glucose cultured cells. Additional treatment of Ferrostatin-1 (Fer-1) in DN models significantly rescued these changes and alleviated the renal pathological injuries in diabetic mice. Besides, the decreased NFE2-related factor 2 (Nrf2) was observed in DN models. The specific knockdown of Nrf2 increased the sensitivity of cells to ferroptosis in the high glucose condition. In Nrf2 knockdown cells, up-regulating Nrf2 by treating with fenofibrate improved the situation of ferroptosis, which was verified in RSL-3 induced cells. Moreover, the ferroptosis-related changes were inhibited by increasing Nrf2 in fenofibrate treated diabetic mice, which delayed the progression of DN. Collectively, we demonstrated that ferroptosis was involved in the development of DN, and up-regulating Nrf2 by treating with fenofibrate inhibited diabetes-related ferroptosis, delaying the progression of DN. Our research revealed the development mechanism of DN from a new perspective, and provide a new approach delaying the progression of DN.
中文翻译:

通过上调 Nrf2 抑制铁死亡延缓糖尿病肾病的进展
糖尿病肾病(DN)现在被认为是终末期肾病的主要原因。在糖尿病中,活性氧(ROS)的积累和铁过载是促进DN发生的重要决定因素。然而,它们如何引起糖尿病肾损害的潜在机制仍不清楚。以铁依赖性脂质过氧化为特征的铁死亡为我们探索DN的进展提供了新思路。在链脲佐菌素诱导的 DBA/2J 糖尿病小鼠和高糖培养的人肾近端小管 (HK-2) 细胞的肾脏中检测到铁过载、抗氧化能力降低、大量 ROS 和脂质过氧化,这是铁死亡的标志性变化。此外,在高糖培养的细胞中观察到铁死亡的特征性线粒体形态变化。在 DN 模型中额外治疗 Ferrostatin-1 (Fer-1) 显着挽救了这些变化并减轻了糖尿病小鼠的肾脏病理损伤。此外,在 DN 模型中观察到 NFE2 相关因子 2 (Nrf2) 降低。Nrf2的特异性敲低增加了细胞在高糖条件下对铁死亡的敏感性。在 Nrf2 敲低细胞中,通过用非诺贝特处理上调 Nrf2 改善了铁死亡的情况,这在 RSL-3 诱导的细胞中得到了证实。此外,在非诺贝特治疗的糖尿病小鼠中,增加 Nrf2 抑制了与铁死亡相关的变化,从而延缓了 DN 的进展。总的来说,我们证明了铁死亡参与了 DN 的发展,并且通过用非诺贝特治疗上调 Nrf2 抑制了糖尿病相关的铁死亡,延缓 DN 的进展。我们的研究从一个新的角度揭示了DN的发展机制,为延缓DN的发展提供了新的途径。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号